Exam 8: Chemical Bonding I: Basic Concepts
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
In which of the following species does the central atom violate the octet rule?
(Multiple Choice)
4.9/5  (30)
(30)
If an element is bonded to 4 other atoms and has a formal charge of +1, what group must the element be in?
(Multiple Choice)
4.9/5  (36)
(36)
The number of resonance structures for the sulfur dioxide molecule that satisfy the octet rule is
(Multiple Choice)
4.9/5  (33)
(33)
In the resonance structure of sulfur dioxide, the sulfur atom has one single bond and one double bond. What is the formal charge of this sulfur atom?
(Multiple Choice)
4.8/5  (35)
(35)
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s). What does the dot or dots represent?
(Multiple Choice)
4.8/5  (31)
(31)
The number of dots in the Lewis dot symbol for a main group element is the same as the ________ ________.
(Short Answer)
4.9/5  (45)
(45)
Which one of the following Lewis structures is definitely incorrect?
(Multiple Choice)
4.9/5  (34)
(34)
In the Lewis dot symbol, the element symbol represents the ________ and the dots represent the ________.
(Short Answer)
4.9/5  (39)
(39)
Select the correct Lewis structure for nitrogen trifluoride, NF3.
(Multiple Choice)
4.8/5  (39)
(39)
The Lewis structure for a chlorate ion, ClO3-, should show ________ single bond(s), ________ double bond(s), and ________ lone pair(s).
(Multiple Choice)
4.8/5  (40)
(40)
What name is given to the sharing of electrons between two atoms?
(Short Answer)
4.8/5  (37)
(37)
How many total resonance structures can be drawn for the sulfate ion, SO42-?
(Multiple Choice)
4.8/5  (40)
(40)
In the following Lewis structure for ClO3F, chlorine has a formal charge of ________ and an oxidation number of ________. 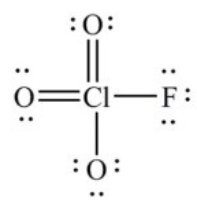
(Multiple Choice)
4.8/5  (34)
(34)
What is the formal charge on phosphorus in a Lewis structure for the phosphate ion that satisfies the octet rule?
(Multiple Choice)
5.0/5  (39)
(39)
In the Lewis structure of the iodate ion, IO3-, that satisfies the octet rule, the formal charge on the central iodine atom is
(Multiple Choice)
4.9/5  (32)
(32)
Showing 41 - 60 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)