Exam 8: Chemical Bonding I: Basic Concepts
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Select the compound with the lowest (i.e., least negative) lattice energy.
(Multiple Choice)
4.8/5  (30)
(30)
Select the correct Lewis structure for NOCl, a reactive material used as an ionizing solvent.
(Multiple Choice)
4.8/5  (34)
(34)
How many dots does the Lewis dot symbol for magnesium have around it?
(Multiple Choice)
4.9/5  (43)
(43)
A double bond cannot exist between a carbon atom and an oxygen atom.
(True/False)
4.9/5  (32)
(32)
Ionic compounds tend to form between metals and nonmetals when electrons are transferred from an element with high ionization energy (metal) to an element with a low electron affinity (nonmetal).
(True/False)
4.8/5  (39)
(39)
How many dots does the Lewis dot symbol for oxygen have around it?
(Multiple Choice)
4.8/5  (34)
(34)
A triple bond arises when two atoms share ________ pairs of electrons.
(Short Answer)
4.7/5  (36)
(36)
A triple bond cannot exist between a carbon atom and a hydrogen atom.
(True/False)
4.7/5  (46)
(46)
Only valence electrons are shown in the Lewis structure held together by covalent bonds.
(True/False)
4.9/5  (40)
(40)
Describe in brief how electronegativity values can be used to predict the percent ionic character of a bond between two atoms.
(Essay)
4.8/5  (45)
(45)
Which of these ionic solids would have the largest lattice energy?
(Multiple Choice)
4.8/5  (36)
(36)
Select the compound with the highest (i.e., most negative) lattice energy.
(Multiple Choice)
4.9/5  (45)
(45)
How many lone pairs of electrons need to be added to complete this Lewis structure? 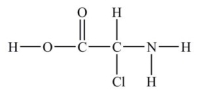
(Multiple Choice)
4.9/5  (37)
(37)
What is the electrostatic attraction called that holds oppositely charged ions together in a compound?
(Short Answer)
4.7/5  (36)
(36)
The formal charge on Cl in the structure shown for the perchlorate ion is ________. 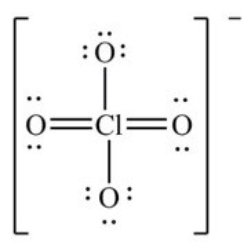
(Multiple Choice)
4.8/5  (35)
(35)
A(n) ________ is a representation of covalent bonding in which shared electron pairs are shown either as dashes or as pairs of dots between two atoms and unshared electrons are shown as dots around the individual atoms.
(Short Answer)
4.9/5  (47)
(47)
Showing 81 - 100 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)