Exam 6: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which scientist proposed that energy of radiation is composed of extremely small indivisible packages called quanta? ("Quanta" is the plural of "quantum.")
(Multiple Choice)
4.9/5  (33)
(33)
Which element has the following ground-state electron configuration? [Kr]5s24d105p3
(Multiple Choice)
5.0/5  (47)
(47)
Select the arrangement of electromagnetic radiation which starts with the lowest energy and increases to the greatest energy.
(Multiple Choice)
4.8/5  (30)
(30)
The orientation in space of an atomic orbital is associated with
(Multiple Choice)
4.8/5  (40)
(40)
The Pauli exclusion principle states that no ________ electrons within an atom can have the same ________ quantum numbers.
(Multiple Choice)
4.9/5  (39)
(39)
For all atoms of the same element, the 2s orbital is larger than the 1s orbital.
(True/False)
4.7/5  (25)
(25)
The electron configuration of a ground-state copper atom is
(Multiple Choice)
4.8/5  (40)
(40)
How many electrons are there in the 2nd principal energy level (n = 2) of a phosphorus atom?
(Multiple Choice)
4.8/5  (39)
(39)
________ is the element represented by the electron configuration [Ne]3s2 3p1.
(Short Answer)
4.8/5  (39)
(39)
What is the correct electron configuration for a germanium (Ge) atom?
(Multiple Choice)
4.8/5  (34)
(34)
The FM station KDUL broadcasts music at 99.1 MHz. Find the wavelength of these waves. (c = 3.00 × 108 m/s)
(Multiple Choice)
4.8/5  (43)
(43)
"No two electrons in an atom can have the same four quantum numbers" is a statement of
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following is a correct set of quantum numbers for an electron in a 5f orbital?
(Multiple Choice)
4.8/5  (37)
(37)
List the following types of radiation from lowest frequency to highest frequency: microwave, X ray, ultraviolet, visible, and infrared
(Multiple Choice)
4.8/5  (37)
(37)
Which one of the following sets of quantum numbers can correctly represent a 3p orbital? 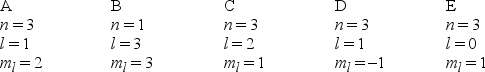
(Multiple Choice)
4.8/5  (39)
(39)
Showing 21 - 40 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)