Exam 6: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
According to ________, no two electrons in an atom can have the same four quantum numbers.
(Short Answer)
4.9/5  (28)
(28)
A possible set of quantum numbers for the last electron added to complete an atom of gallium (Ga) in its ground state is 
(Multiple Choice)
4.9/5  (36)
(36)
When a solid is heated, it emits electromagnetic radiation known as ________. An example of such radiation is the element of a stove stop burning bright red.
(Multiple Choice)
4.8/5  (31)
(31)
________ is the wavelength associated with a moving particle.
(Multiple Choice)
4.9/5  (35)
(35)
The ground-state electron configuration for an atom of indium is
(Multiple Choice)
4.9/5  (33)
(33)
The maximum number of electrons that can occupy an energy level described by the principal quantum number, n, is
(Multiple Choice)
4.7/5  (32)
(32)
An electron in a 3p orbital could have a value of 2 for its angular momentum quantum number (l).
(True/False)
4.9/5  (34)
(34)
What is the wavelength of light having a frequency of 4.8 × 1014 s-1? (c = 3.00 x 108 m/s)
(Multiple Choice)
4.9/5  (39)
(39)
What is the energy in joules of a mole of photons associated with red light of wavelength 7.00 × 102 nm? (c = 3.00 × 108 m/s; h = 6.63 × 10-34 J • s; NA = 6.022 × 1023 /mole)
(Multiple Choice)
4.9/5  (26)
(26)
Which element has the following ground-state electron configuration? 1s2 2s2 2p6 3s2
(Multiple Choice)
4.9/5  (38)
(38)
Line spectra from all regions of the electromagnetic spectrum, including the Paschen series of infrared lines for hydrogen, are used by astronomers to identify elements present in the atmospheres of stars. Calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3. (R = 1.096776 × 107 m-1)
(Multiple Choice)
4.8/5  (45)
(45)
In not more than three lines for each answer, briefly outline one important scientific contribution of each of the following.
a) Planck
b) De Broglie
c) Heisenberg
(Essay)
4.9/5  (39)
(39)
According to scientist James Clerk Maxwell in the year 1873, a(n) ________ consists of an electric field component and a magnetic field component.
(Multiple Choice)
4.8/5  (36)
(36)
Each shell (principal energy level) of quantum number n contains n subshells.
(True/False)
5.0/5  (26)
(26)
________ is the number of subshells in the shell designated as n = 2.
(Short Answer)
4.8/5  (33)
(33)
If one s orbital were combined with one p orbital (dumbbell shaped), which would best describe the resulting shape?
(Multiple Choice)
5.0/5  (40)
(40)
In the quantum mechanical treatment of the hydrogen atom, which one of the following combinations of quantum numbers is not allowed? 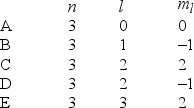
(Multiple Choice)
4.9/5  (36)
(36)
Showing 81 - 100 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)