Exam 6: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
How many orbitals are allowed in a subshell if the angular momentum quantum number for electrons in that subshell is 3?
(Multiple Choice)
4.8/5  (44)
(44)
Which ground-state atom has an electron configuration described by the following orbital diagram? 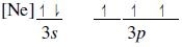
(Multiple Choice)
4.8/5  (39)
(39)
________ is the number of waves passing through a specific point per second.
(Short Answer)
4.9/5  (44)
(44)
The most stable arrangement of electrons in orbitals of equal energy is the one in which the number of electrons with the same spin is maximized is
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following subshells has the highest energy in the element tantalum?
(Multiple Choice)
4.8/5  (40)
(40)
The de Broglie equation predicts that the wavelength (in m) of a proton moving at 1000. m/s is ________. (h = 6.63 × 10-34 J • s; mass of a proton = 1.673 × 10-24 g)
(Multiple Choice)
4.9/5  (39)
(39)
How many electrons are there in the 3rd principal energy level (n = 3) of a phosphorus atom?
(Multiple Choice)
4.8/5  (37)
(37)
A possible set of quantum numbers for the last electron added to complete an atom of germanium in its ground state is 
(Multiple Choice)
5.0/5  (39)
(39)
The solar radiation spectrum peaks at a wavelength of approximately 500 nm. Calculate the energy of one photon of that radiation (c = 3.00 × 108 m/s; h = 6.63 × 10-34 J • s).
(Multiple Choice)
5.0/5  (39)
(39)
How many orbitals are there in the n = 4 level of the H-atom?
(Multiple Choice)
4.8/5  (35)
(35)
Select the arrangement of electromagnetic radiation which starts with the shortest wavelength and increases to longest wavelength.
(Multiple Choice)
4.8/5  (42)
(42)
In which region of the electromagnetic spectrum would radiation of the wavelength λ = 147.3 nm be found?
(Short Answer)
4.8/5  (40)
(40)
The electron configuration of a ground-state vanadium atom is
(Multiple Choice)
4.8/5  (36)
(36)
What is the total number of electrons that can occupy the 4f orbitals?
(Short Answer)
4.8/5  (43)
(43)
What is the maximum number of electrons in an atom that can have the following set of quantum numbers? n = 4, l = 3, ml = -2, ms = +1/2
(Multiple Choice)
4.9/5  (32)
(32)
Calculate the wavelength, in nanometers, of the light emitted by a hydrogen atom when its electron drops from the n = 7 to the n = 4 principal energy level. Recall that the energy levels of the H atom are given by En = -2.18 × 10-18 J (1/n2). (c = 3.00 × 108 m/s; h = 6.63 × 10-34 J • s)
(Multiple Choice)
4.8/5  (29)
(29)
________ is the quantum number which describes the shape of an atomic orbital.
(Short Answer)
4.8/5  (26)
(26)
Which element has the following ground-state electron configuration? [Kr]5s24d105p2
(Multiple Choice)
4.9/5  (43)
(43)
For the following equations
a. name the scientist to whom the equation is attributed.
b. in not more than three lines, explain clearly what the equation means or represents.
1. E = nhv
2. λ = h/mu
(Essay)
4.8/5  (35)
(35)
Showing 61 - 80 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)