Exam 14: Electron Flow in Organotrophy, Lithotrophy, and Phototrophy
Exam 1: Microbial Life: Origin and Discovery70 Questions
Exam 2: Observing the Microbial Cell69 Questions
Exam 3: Cell Structure and Function72 Questions
Exam 4: Bacterial Culture, Growth, and Development70 Questions
Exam 5: Environmental Influences and Control of Microbial Growth70 Questions
Exam 6: Viruses70 Questions
Exam 7: Genomes and Chromosomes70 Questions
Exam 8: Transcription, Translation, and Bioinformatics76 Questions
Exam 9: Gene Transfer, Mutations, and Genome Evolution72 Questions
Exam 10: Molecular Regulation73 Questions
Exam 11: Viral Molecular Biology70 Questions
Exam 12: Biotechniques and Synthetic Biology72 Questions
Exam 13: Energetics and Catabolism77 Questions
Exam 14: Electron Flow in Organotrophy, Lithotrophy, and Phototrophy73 Questions
Exam 15: Biosynthesis73 Questions
Exam 16: Food and Industrial Microbiology73 Questions
Exam 17: Origins and Evolution70 Questions
Exam 18: Bacterial Diversity71 Questions
Exam 19: Archaeal Diversity70 Questions
Exam 20: Eukaryotic Diversity69 Questions
Exam 21: Microbial Ecology70 Questions
Exam 22: Microbes in Global Elemental Cycles70 Questions
Exam 23: Human Microbiota and Innate Immunity70 Questions
Exam 24: The Adaptive Immune Response70 Questions
Exam 25: Microbial Pathogenesis70 Questions
Exam 26: Microbial Diseases69 Questions
Exam 27: Antimicrobial Therapy72 Questions
Exam 28: Clinical Microbiology and Epidemiology75 Questions
Select questions type
Which of the following is FALSE with respect to the use of sulfate by marine archaeal and bacterial groups?
(Multiple Choice)
4.9/5  (42)
(42)
How does Shewanella oneidensis donate electrons to oxidized minerals in marine sediments?
(Essay)
4.9/5  (49)
(49)
Which organism can respire with uranium and remediate uranium-contaminated water?
(Multiple Choice)
4.9/5  (40)
(40)
The figure below shows anaerobic corrosion of a piece of steel accelerated by the action of anaerobic microorganisms. What should be on label 2? 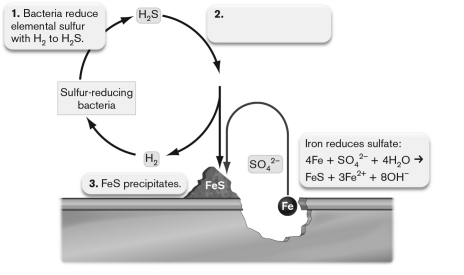 a. Elemental sulfur and iron directly react to produce .
b. Iron reduces
c. Acid rain is produced:
d. Acid rain is produced:
e. Acid rain is produced:
a. Elemental sulfur and iron directly react to produce .
b. Iron reduces
c. Acid rain is produced:
d. Acid rain is produced:
e. Acid rain is produced:
(Short Answer)
4.9/5  (41)
(41)
Although embedded in the membrane as an ETS component, the enzyme __________ does not pump protons.
(Multiple Choice)
4.9/5  (30)
(30)
Standard reduction potentials and standard free energy change are related by the
equation , where F is the Faraday’s constant and n is the number of electrons
transferred from donor to acceptor. has units of kJ/mol. Which of the following redox pairs
will have the highest value?
(1)
(2)
(Essay)
4.9/5  (34)
(34)
The molecule shown below, __________, is a cofactor for electron transport. 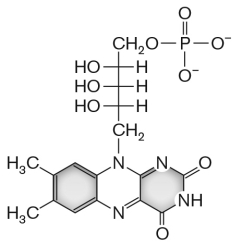
(Multiple Choice)
4.9/5  (35)
(35)
Electron donors for anaerobic photosystem I in green sulfur bacteria and chloroflexi include the following, EXCEPT
(Multiple Choice)
4.8/5  (34)
(34)
In photosystem II of purple bacteria, electrons flow through cytochrome bc, coupled to pumping of protons and the proton potential drives synthesis of ATP. The cytochrome bc complex transfers the electrons back to bacteriochlorophyll P870. This type of ATP synthesis is called
(Multiple Choice)
4.9/5  (41)
(41)
Use the figure below to describe Peter Mitchell's chemiosmotic model for coupling the electron transfer system with ATP synthesis.
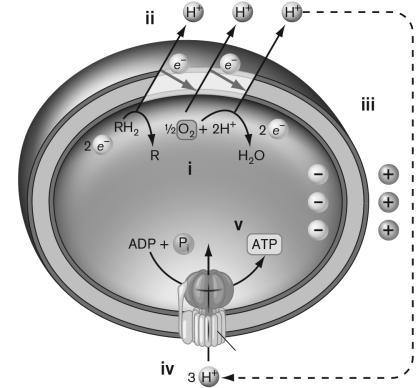
(Essay)
4.8/5  (43)
(43)
Showing 61 - 73 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)