Exam 12: Intracellular Compartments and Protein Sorting
Exam 1: Cells and Genomes34 Questions
Exam 2: Cell Chemistry and Bioenergetics54 Questions
Exam 3: Proteins52 Questions
Exam 4: DNA, Chromosomes, and Genomes57 Questions
Exam 5: DNA Replication, Repair, and Recombination51 Questions
Exam 6: How Cells Read the Genome: From DNA to Protein58 Questions
Exam 7: Control of Gene Expression62 Questions
Exam 8: Analyzing Cells, Molecules, and Systems95 Questions
Exam 9: Visualizing Cells29 Questions
Exam 10: Membrane Structure26 Questions
Exam 11: Membrane Transport of Small Molecules and the Electrical Properties of Membranes46 Questions
Exam 12: Intracellular Compartments and Protein Sorting46 Questions
Exam 13: Intracellular Membrane Traffic54 Questions
Exam 14: Energy Conversion: Mitochondria and Chloroplasts49 Questions
Exam 15: Cell Signaling63 Questions
Exam 16: The Cytoskeleton75 Questions
Exam 17: The Cell Cycle57 Questions
Exam 18: Cell Death12 Questions
Exam 19: Cell Junctions and the Extracellular Matrix56 Questions
Exam 20: Cancer50 Questions
Exam 21: Development of Multicellular Organisms61 Questions
Exam 22: Stem Cells and Tissue Renewal45 Questions
Exam 23: Pathogens and Infection32 Questions
Exam 24: The Innate and Adaptive Immune Systems47 Questions
Select questions type
Indicate whether each of the following occurs on the cytosolic side of the ER membrane (C), on the lumenal side of the ER membrane (L), or in the Golgi apparatus (G). Your answer would be a five-letter string composed of letters C, L, and G only, e.g. GGGLC.
( ) Assembly of GlcNAc oligosaccharides on dolichol phosphate
( ) Synthesis of phosphatidylcholine
( ) Synthesis of sphingomyelin
( ) Ubiquitylation of misfolded ER protein
( ) Synthesis of phosphatidic acid
Free
(Short Answer)
4.8/5  (26)
(26)
Correct Answer:
C
C
G
C
C
Considering the following hydropathy plot for a multipass transmembrane protein in the plasma membrane, are the protein termini expected to be located inside (I) or outside (O) of the cell? Which of the regions indicated as 2 and 3 in the plot is expected to have more positively charged residues? The position of positively charged residues near the first transmembrane helix is indicated by an asterisk on the plot. 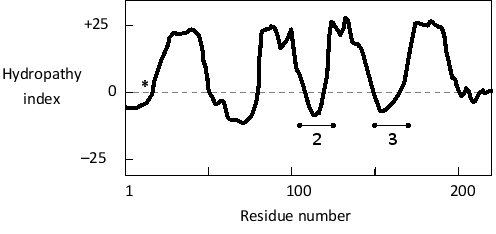
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
A
Mitochondrial hsp70 is to matrix protein import what ... is to post-translational ER protein import.
Free
(Multiple Choice)
4.7/5  (42)
(42)
Correct Answer:
D
Indicate true (T) and false (F) statements below regarding the mitochondrial protein import system. Your answer would be a four-letter string composed of letters T and F only, e.g. TTTF.
( ) Mitochondrial proteins should fold natively twice: once in the cytosol and once inside the organelle.
( ) ?-barrel proteins that are abundant in the mitochondrial outer membrane are imported from the cytosol independently of the TOM complex.
( ) Signal sequences that target precursor proteins to the mitochondrial matrix form an ?- helix in which positively charged residues cluster near its N-terminus, while uncharged or hydrophobic residues cluster near the other side.
( ) At least two signal sequences are required to direct proteins to the mitochondrial matrix.
(Short Answer)
4.9/5  (32)
(32)
Consider a transcription regulatory protein that has both a nuclear localization and a nuclear export signal and is normally found both in the nucleus and in the cytosol at comparable concentrations. This protein has a high-affinity binding partner in the nucleus. Upon activation of a certain signaling pathway, the binding protein is ubiquitylated and degraded. As a result of this, ...
(Multiple Choice)
4.8/5  (42)
(42)
Certain cysteine-containing proteins of the mitochondrial intermembrane space are imported from the cytosol with the help of the Mia40 protein via a disulfide relay system. What drives the unidirectional import of these proteins? Are these proteins reduced or oxidized at their cysteine residues upon import?
(Multiple Choice)
4.7/5  (27)
(27)
A geneticist has devised a strategy to study protein translocation into the endoplasmic reticulum (ER) in yeast cells. She is interested in two different signal sequences that are thought to operate via slightly different translocation mechanisms. Using genetic engineering, she has fused the first signal sequence to a protein whose cytosolic expression is absolutely necessary for cell survival in the selective medium, but is inactive when in the ER. In the same cell, she has also fused the second signal sequence to a toxic protein whose cytosolic expression leads to cell lysis but is harmless when in the ER. Whereas wild-type cells undergo lysis upon the expression of these fusion proteins, she has been able to identify viable mutants, each of which has a loss-of-function mutation in a gene encoding a protein involved in membrane translocation. The products of these genes are probably ...
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is NOT a likely ER signal sequence recognized by the signal-recognition particle? All sequences are written with their N-terminus on the left.
(Multiple Choice)
4.8/5  (39)
(39)
The formation of a stable ternary complex involving Ran GTPase, a nuclear transport receptor, and a cargo protein occurs in ...
(Multiple Choice)
4.9/5  (31)
(31)
Indicate true (T) and false (F) statements below regarding peroxisomal proteins. Your answer would be a four-letter string composed of letters T and F only, e.g. TTTF.
( ) All peroxisomal proteins are encoded in the nucleus.
( ) Some peroxisomal proteins are synthesized by ribosomes attached to the rough endoplasmic reticulum.
( ) All peroxisomal proteins reach the organelle after their synthesis is completed.
( ) Peroxisomal proteins have to be unfolded before import.
(Short Answer)
4.8/5  (29)
(29)
Which of the following scenarios does NOT normally occur on a nuclear pore complex?
(Multiple Choice)
4.8/5  (42)
(42)
Which subset of the following is directly involved in driving protein import into the mitochondrial matrix space? Choose all correct sources. Your answer would be a string composed of letters A to G only, in alphabetical order, e.g. AE.
A. ATP hydrolysis inside mitochondria
B. ATP hydrolysis outside mitochondria
C. GTP hydrolysis inside mitochondria
D. GTP hydrolysis outside mitochondria
E. Light
F. Membrane potential across the inner membrane
G. Membrane potential across the outer membrane
(Short Answer)
5.0/5  (39)
(39)
Proteins of the protein disulfide isomerase (PDI) family share common domains that harbor an active-site CXXC motif (C = cysteine; X = one of several residues). During the course of a redox reaction, one of the active-site cysteines in its reduced form can attack a disulfide bond in a substrate protein (that could itself be another PDI family member) to form a mixed disulfide between the enzyme and its substrate. This is then attacked by the other active-site cysteine, releasing the substrate in reduced form. The reverse of these reactions can occur instead in order to make disulfide bonds in target proteins. CXXA mutant PDI family proteins-in which one of the active-site cysteines is mutated to an alanine-can be trapped in the intermediate mixed disulfide state for an elongated time, facilitating the identification of their substrate proteins. Using these mutations in each of three interacting PDI family proteins-A, B, and C (one mutation at a time)-you have discovered that the mutant B interacts with A and C, while mutant A interacts with C but not with B. Finally, mutant C interacts with neither of the other two. The following diagram shows the thermodynamically favorable flow of electrons (plus protons) in the cascade involving these three proteins. Based on your results above, what proteins correspond to 1, 2, and 3 in the diagram, respectively? Your answer would be a three-letter string composed of letters A to C only, e.g. CBA.
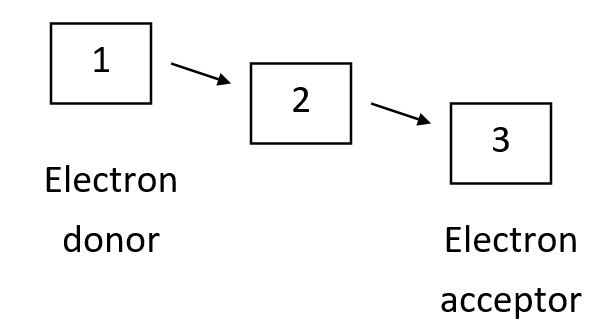
(Short Answer)
4.9/5  (28)
(28)
A protein is covalently attached to glycosylphosphatidylinositol. Which of the following is typically NOT true regarding this protein?
(Multiple Choice)
4.7/5  (31)
(31)
In the following schematic diagram of a nuclear pore complex, on which side is the cytosol located? What is the approximate diameter of the pore, as indicated by the vertical bar? 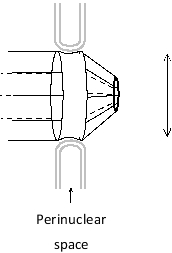
(Multiple Choice)
4.9/5  (38)
(38)
Consider a human liver hepatocyte. Among the following membranes, which one has the largest total area?
(Multiple Choice)
4.9/5  (33)
(33)
Indicate whether the location of each of the following is topologically equivalent to the cytosol (C) or the extracellular space (E). Your answer would be a five-letter string composed of letters C and E only, e.g. CCECE.
( ) Ribosomes
( ) Chromatin
( ) Lysosomal hydrolases
( ) Calcium ions in the ER
( ) Peroxisomal catalase
(Short Answer)
4.8/5  (36)
(36)
Fill in the blank in the following paragraph regarding the recognition of tail-anchored proteins by their targeting machinery. DO NOT use abbreviations.
"When the hydrophobic transmembrane ? helix at the C-terminus of an ER tail-anchored protein emerges from the ribosome, the tail-anchored-binding domain (TABD) of the Get3 ATPase binds to it. The job of TABD in Get3 is similar to that of the flexible hydrophobic domain in the SRP54 protein of the signal-recognition particle, in that it also has to recognize a degenerate set of hydrophobic ?- helices. Therefore, it is not surprising that, just like the corresponding domain in SRP54, the binding site in Get3 is rich in ... residues."
(Short Answer)
4.9/5  (32)
(32)
Compared to the cytosol, which of the following is generally true about the lumen of the endoplasmic reticulum in our cells?
(Multiple Choice)
4.9/5  (35)
(35)
Cyclin B1, a key cell cycle regulatory protein in vertebrates, is mostly cytosolic before mitosis. Early in mitosis, however, the protein is phosphorylated by certain protein kinases and consequently accumulates in the nucleus. How can phosphorylation bring about nuclear accumulation of this protein?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)