Exam 12: Intracellular Compartments and Protein Sorting
Exam 1: Cells and Genomes34 Questions
Exam 2: Cell Chemistry and Bioenergetics54 Questions
Exam 3: Proteins52 Questions
Exam 4: DNA, Chromosomes, and Genomes57 Questions
Exam 5: DNA Replication, Repair, and Recombination51 Questions
Exam 6: How Cells Read the Genome: From DNA to Protein58 Questions
Exam 7: Control of Gene Expression62 Questions
Exam 8: Analyzing Cells, Molecules, and Systems95 Questions
Exam 9: Visualizing Cells29 Questions
Exam 10: Membrane Structure26 Questions
Exam 11: Membrane Transport of Small Molecules and the Electrical Properties of Membranes46 Questions
Exam 12: Intracellular Compartments and Protein Sorting46 Questions
Exam 13: Intracellular Membrane Traffic54 Questions
Exam 14: Energy Conversion: Mitochondria and Chloroplasts49 Questions
Exam 15: Cell Signaling63 Questions
Exam 16: The Cytoskeleton75 Questions
Exam 17: The Cell Cycle57 Questions
Exam 18: Cell Death12 Questions
Exam 19: Cell Junctions and the Extracellular Matrix56 Questions
Exam 20: Cancer50 Questions
Exam 21: Development of Multicellular Organisms61 Questions
Exam 22: Stem Cells and Tissue Renewal45 Questions
Exam 23: Pathogens and Infection32 Questions
Exam 24: The Innate and Adaptive Immune Systems47 Questions
Select questions type
Consider two cells, A and B. Both are approximately spherical, but cell A is a bacterium with a diameter of only about 1 µm, while the diameter of the eukaryotic cell B is about 10 µm. If the plasma membrane in the eukaryotic cell comprises only about 2% of the total cell membrane, which cell has a higher ratio of total cell membrane to volume? Write down A or B as your answer.
(Short Answer)
4.9/5  (42)
(42)
Tom40 is a nuclear-encoded essential subunit of the TOM complex in the outer mitochondrial membrane. It is a ?-barrel protein that forms the pore through which precursor proteins enter the intermembrane space from the cytosol. Indicate true (T) and false (F) statements below regarding the Tom40 protein. Your answer would be a four-letter string composed of letters T and F only, e.g. TTTF.
( ) Incorporation of new Tom40 in the outer membrane requires preexisting Tom40 in that membrane.
( ) Formation of new TOM complexes is dependent on the SAM complex.
( ) Tom40 is partially translocated through the outer membrane and is then transferred in the plane of the membrane to fold into its native conformation.
( ) Tom40 is translocated through the inner membrane as a precursor.
(Short Answer)
4.9/5  (34)
(34)
Fill in the blank in the following paragraph. DO NOT use abbreviations.
"A significant fraction of total membrane area in a eukaryotic cell encloses the lumen of the ..., which forms an extended netlike labyrinth of tubules and sacs. Secretory proteins are normally synthesized by ribosomes bound to a special type of this compartment."
(Short Answer)
4.9/5  (32)
(32)
Indicate whether each of the following descriptions better matches the ATF6 (A), the IRE1 (I), or the PERK (P) branch of the ER unfolded protein response. Your answer would be a four-letter string composed of letters A, I, and P only, e.g. APII.
( ) It involves a noncanonical cytoplasmic splicing process.
( ) Its sensor is a latent transcription regulator.
( ) It involves regulated proteolysis of the sensor protein in the Golgi apparatus.
( ) Its sensor bears both kinase and endoribonuclease activities.
(Short Answer)
4.8/5  (38)
(38)
You set up an in vitro translation system containing the entire translation machinery but devoid of any component of the endoplasmic reticulum (ER) targeting machinery. To this system, you can add mRNA encoding either a 20 kD secretory protein or a 20 kD cytosolic protein. You perform in vitro translation in the presence of radioactively labeled methionine, with or without the addition of saturating amounts of SRP or microsomes, as indicated below. After separating the protein products by SDS-PAGE, and visualizing the radioactivity by autoradiography, you obtain the following results. The presence or absence of each component in the reaction is indicated at the top of the corresponding lane(s) by + and -, respectively. The numbers on the left indicate the apparent molecular mass (×1000) of spots on the gel. Which protein (X or Y) is the secretory protein? Which of the reactions (1 or 2) contained SRP? 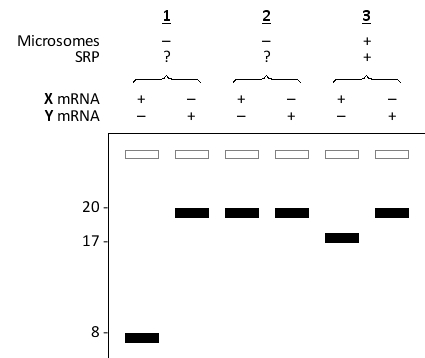
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following proteins or protein complexes is directly required for the targeting of mitochondrial inner membrane multipass proteins, such as metabolite transporters, whose signal sequence is normally not cleaved after import?
(Multiple Choice)
4.9/5  (35)
(35)
Misfolded proteins in the ER may actively undergo any of the following EXCEPT ...
(Multiple Choice)
4.7/5  (35)
(35)
Indicate whether each of the following descriptions refers to protein import into mitochondria (M), chloroplasts (C), or both (B). Your answer would be a five-letter string composed of letters M, C, and B only, e.g. BBBMC.
( ) ATP and GTP hydrolysis drive translocation into the organelle.
( ) The organelle has an extra compartment that requires extra signal sequences for protein targeting.
( ) Transport through the double membrane is driven in part by an H? gradient across the inner membrane.
( ) Imported precursor proteins have amphiphilic N-terminal signal sequences that are usually removed after use.
( ) Hsp70 family chaperones inside the organelle assist in protein translocation during import.
(Short Answer)
4.8/5  (31)
(31)
In the following graph, the magnitude of concentration difference across the nuclear pore complexes (NPCs) is plotted for four molecules (A to D) as a function of time, starting from an arbitrarily chosen initial concentration difference. Indicate which curve corresponds to each of the following molecules. Your answer would be a four-letter string composed of letters A to D only, e.g. ABCD.
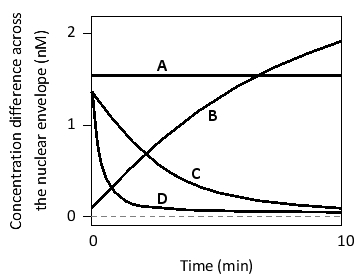 ( ) A large protein that is being actively transported across the NPC
( ) A small water-soluble molecule
( ) A small protein composed of a few dozen residues
( ) A large protein that is NOT actively transported into or out of the nucleus
( ) A large protein that is being actively transported across the NPC
( ) A small water-soluble molecule
( ) A small protein composed of a few dozen residues
( ) A large protein that is NOT actively transported into or out of the nucleus
(Short Answer)
4.8/5  (29)
(29)
The signal-recognition particle is not the only factor that evaluates the authenticity of endoplasmic reticulum (ER) signal sequences in proteins. The Sec61 complex is also able to recognize the signal sequences and "opens" after binding to them. Even single point mutations within the signal sequence of a protein can render the protein unable to enter the ER efficiently, as the Sec61 complex does not readily open in response to the mutant sequence. However, "suppressor" mutations in genes encoding components of the translocation pathway, including the Sec61 subunits, can partially restore the wild-type localization of proteins with mutant signal sequences. Many such suppressor mutations (also called prl mutations) map to or near the "plug" domain in the Sec61 translocon. These mutations, including the deletion of the entire plug, generally result in destabilization of the closed conformation of the translocon and favor its open conformation. Your friend has mutated a certain residue in the plug domain of the yeast Sec61. She has just finished measuring the translocation efficiency of an ER protein with either a wild-type or a mutant (defective) signal sequence, either in wild-type or in her Sec61-mutant cells, and has obtained the following results. Based on these early results, does her Sec61 mutation show a prl phenotype? Write down Yes or No as your answer.
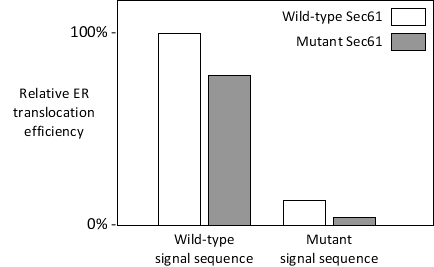
(Short Answer)
4.8/5  (31)
(31)
Indicate true (T) and false (F) statements below regarding the compartmentalization of cells. Your answer would be a four-letter string composed of letters T and F only, e.g. TTTF.
( ) Almost all eukaryotic cells have plastids, but only plant cells have chloroplasts capable of photosynthesis.
( ) The perinuclear space is topologically equivalent to the extracellular space.
( ) The mitochondria and chloroplasts are thought to have evolved by invagination and pinching off from the plasma membrane of the ancient eukaryotic cell.
( ) All organelles in a eukaryotic cell can be constructed de novo, which means that the information to construct them is encoded in the genome.
(Short Answer)
4.8/5  (41)
(41)
Rough microsomes can be subjected to a "salt extraction" procedure in which a high salt concentration is used to remove membrane-associated ribosomes and peripheral proteins. Such salt-extracted microsomes are known to be translocation-incompetent, meaning that when present co-translationally in vitro, they fail to protect translated proteins from protease digestion. However, adding back an 11S particle (S is the sedimentation coefficient) purified from the salt-wash fraction is sufficient to restore the protein-translocation activity of the salt-extracted microsomes. Which of the following do you think is true regarding the 11S particle?
(Multiple Choice)
4.8/5  (36)
(36)
According to the model for nuclear transport described in this chapter, what do you think would happen if you could artificially limit all Ran-GAP activity to the nucleus and all Ran-GEF activity to the cytosol?
(Multiple Choice)
4.9/5  (30)
(30)
Indicate true (T) and false (F) statements below regarding N-linked glycosylation of proteins. Your answer would be a four-letter string composed of letters T and F only, e.g. TTTF.
( ) N-linked glycosylation can be carried out co-translationally, possibly at multiple asparagine residues on the same protein molecule.
( ) N-linked glycosylation is a gradual process, with step-by-step addition and trimming events that commence with the addition of N-acetylglucosamine to an asparagine side chain.
( ) Most proteins synthesized in the rough ER are N-glycosylated, and some of them require this modification for their correct folding.
( ) Once a protein is properly folded in the ER, its attached oligosaccharides are quickly removed by an N-glycanase, although it may be glycosylated again later.
(Short Answer)
4.8/5  (38)
(38)
The following diagram depicts the topology of a multipass transmembrane protein in the endoplasmic reticulum (ER) membrane. Which set of helices act as stop-transfer signals in this protein? 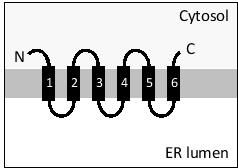
(Multiple Choice)
4.8/5  (33)
(33)
Indicate whether the C-terminus (C) or the N-terminus (N) of each of the following proteins is expected to be located in the cytosol upon membrane integration of the protein. Your answer would be a three-letter string composed of letters C and N only, e.g. CCC.
( ) A single-pass transmembrane protein that has one N-terminal signal sequence and one internal stop-transfer signal
( ) A single-pass transmembrane protein that has one internal signal sequence that is preceded by a patch of positively charged residues
( ) An ER tail-anchored protein
(Short Answer)
4.9/5  (32)
(32)
Sort the following events as they occur during the peroxisomal protein import cycle, starting with the release of cargo from Pex5. Your answer would be a five-letter string composed of letters A to E only, e.g. ABEDC.
(A) Pex5 deubiquitylation
(B) Pex5 ubiquitylation
(C) Docking and translocation of the cargo protein along with Pex5
(D) Pex5 export from the peroxisome with the help of ATPases Pex1 and Pex6
(E) Pex5 binding to a cargo protein containing a C-terminal peroxisomal targeting sequence
(Short Answer)
4.9/5  (37)
(37)
Consider a transmembrane protein with the following topology that has an internal signal sequence (helix 1). If you fuse a canonical ER signal sequence at the N-terminus of this protein, how would you expect the topology to change? The ER lumen is at the bottom in all drawings. For simplicity, assume that the effect of charged residues flanking the transmembrane helices is negligible in this case. 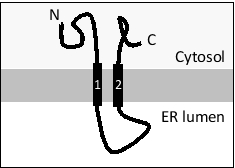
(Multiple Choice)
4.8/5  (38)
(38)
Indicate true (T) and false (F) statements below regarding the nuclear transport of proteins. Your answer would be a four-letter string composed of letters T and F only, e.g. TTTF.
( ) Many nuclear import and export receptors are members of the same protein family.
( ) Most nuclear import receptors contain unstructured domains with FG-repeats.
( ) Adaptor proteins that simultaneously bind to nuclear localization signals and to importins are required for the import of some nuclear cargo proteins.
( ) Ran is mostly found in its GTP-bound form in the nucleus.
(Short Answer)
5.0/5  (40)
(40)
Showing 21 - 40 of 46
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)