Exam 16: The Cytoskeleton
Exam 1: Cells and Genomes34 Questions
Exam 2: Cell Chemistry and Bioenergetics54 Questions
Exam 3: Proteins52 Questions
Exam 4: DNA, Chromosomes, and Genomes57 Questions
Exam 5: DNA Replication, Repair, and Recombination51 Questions
Exam 6: How Cells Read the Genome: From DNA to Protein58 Questions
Exam 7: Control of Gene Expression62 Questions
Exam 8: Analyzing Cells, Molecules, and Systems95 Questions
Exam 9: Visualizing Cells29 Questions
Exam 10: Membrane Structure26 Questions
Exam 11: Membrane Transport of Small Molecules and the Electrical Properties of Membranes46 Questions
Exam 12: Intracellular Compartments and Protein Sorting46 Questions
Exam 13: Intracellular Membrane Traffic54 Questions
Exam 14: Energy Conversion: Mitochondria and Chloroplasts49 Questions
Exam 15: Cell Signaling63 Questions
Exam 16: The Cytoskeleton75 Questions
Exam 17: The Cell Cycle57 Questions
Exam 18: Cell Death12 Questions
Exam 19: Cell Junctions and the Extracellular Matrix56 Questions
Exam 20: Cancer50 Questions
Exam 21: Development of Multicellular Organisms61 Questions
Exam 22: Stem Cells and Tissue Renewal45 Questions
Exam 23: Pathogens and Infection32 Questions
Exam 24: The Innate and Adaptive Immune Systems47 Questions
Select questions type
In the following graph that shows changes in the lengths of two microtubules over time, which time point corresponds to a catastrophe for both microtubules? Which trace corresponds to a microtubule with greater dynamic instability? 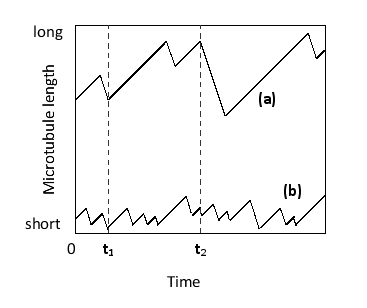
(Multiple Choice)
4.9/5  (28)
(28)
A microtubule appears as a left-handed helix due to an approximately 0.9-nm stagger in the lateral contacts between adjacent protofilaments. In the lateral contacts, α- and β-tubulins in one protofilament interact with α- and β-tubulins, respectively, in the neighboring protofilament, except for a longitudinal discontinuity along the microtubule called the seam. Along the seam, lateral contacts have to be made between different tubulins (i.e. α-tubulin with β-tubulin). Which of the following do you think is acceptable as the repeat distance of tubulin monomers along a protofilament?
(Multiple Choice)
4.7/5  (43)
(43)
If myosin II heads are attached to a glass slide and actin filaments are allowed to bind to them, the filaments will glide on the surface …
(Multiple Choice)
4.9/5  (32)
(32)
According to the following graph, which shows the elongation rate at the plus and minus ends of actin filaments as a function of actin subunit concentration, at what concentration (A to E) does the total length of the filament remain more or less constant with time (i.e. steady-state treadmilling occurs)? 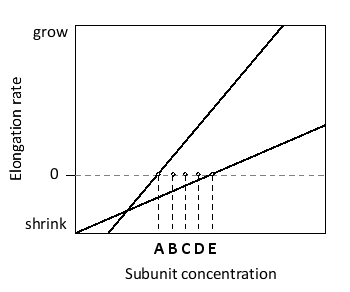
(Short Answer)
4.9/5  (43)
(43)
Cofilin binds preferentially to ADP-containing actin filaments rather than to ATP-containing filaments. Consequently, this protein …
(Multiple Choice)
4.8/5  (32)
(32)
In the dendritic networks of actin filaments in lamellipodia, nucleation of actin polymerization is mostly performed by …
(Multiple Choice)
4.8/5  (39)
(39)
Indicate whether each of the following descriptions applies to myosins (M), kinesins (K), or dyneins (D). Your answer would be a five-letter string composed of letters M, K, and D only, e.g. MMMDD.
( ) They have larger structures than the other two.
( ) They are generally faster than the other two.
( ) They are structurally unrelated to the other two.
( ) They walk on a different cytoskeletal filament than the other two.
( ) They are all minus-end directed.
(Short Answer)
4.8/5  (34)
(34)
Indicate whether each of the following descriptions applies to cilia (C), flagella (F), or both (B). Your answer would be a four-letter string composed of letters C, F, and B only, e.g. CCFB.
( ) They are short and present at high numbers per cell.
( ) They have a whiplike motion that resembles breaststroke in swimming.
( ) They are based on the axoneme structure.
( ) They are found in the epithelial cells of the human respiratory tract.
(Short Answer)
4.8/5  (37)
(37)
A dimeric kinesin-1 molecule has 8-nm steps and can move at rates of about 1 µm/sec. Olympic 100-meter sprinters typically run at about 180 steps per minute and can reach speeds of about 42 km/h. With the same step size, if the Olympic runner had a step frequency of a kinesin-1 molecule, how fast could she run? Write down your answer in km/h, e.g. 52 km/h.
(Short Answer)
4.9/5  (41)
(41)
Which of the following actin-binding proteins cannot bind to the same actin filament simultaneously?
(Multiple Choice)
4.9/5  (48)
(48)
"Headless" kinesin mutants only contain the stalk (middle) and tail domains and can therefore dimerize with their wild-type kinesin partners. However, since they lack the motor (head) domain, the resulting dimers are unable to carry out processive transport of their cargoes and the mutation thus behaves as "dominant negative," meaning that the mutant not only is nonfunctional, but can also interfere with the function of its wild-type counterparts. If a headless mutant of a kinesin heavy chain involved in melanosome movement is overexpressed in fish melanocytes, what would you predict happens in these cells?
(Multiple Choice)
4.7/5  (41)
(41)
In the following graph of actin elongation rates under different subunit concentrations, which of the following corresponds to the slope of the line? 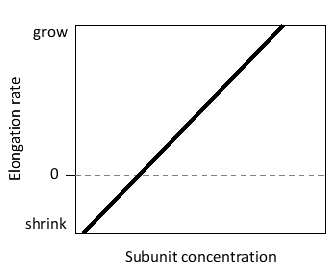
(Multiple Choice)
4.8/5  (35)
(35)
Consider an actin subunit that has just been incorporated into an actin filament at the leading edge of a lamellipodium. Before its ATP is hydrolyzed, how does its distance from the leading front edge of the plasma membrane change over time? How does its distance from the F-actin minus end change over time?
(Multiple Choice)
4.9/5  (35)
(35)
In the presence of an ATP analog that can bind myosin normally but cannot be hydrolyzed, …
(Multiple Choice)
4.7/5  (45)
(45)
Showing 61 - 75 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)