Exam 2: Atoms and Molecules
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
Which of the following correctly describes subatomic particles? 1. Mass: 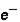 <
< 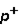 = n
2. Magnitude of charge: n
= n
2. Magnitude of charge: n 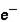 =
= 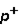 3. Location: outside nucleus
3. Location: outside nucleus 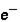 ,
, 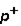 , inside nucleus n
, inside nucleus n
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following units is the relative mass of a molecule expressed in atomic mass units and is calculated by adding together the atomic weights of the atoms in the molecule?
(Multiple Choice)
4.8/5  (37)
(37)
What is wrong with the following molecular formula: SOO (sulfur dioxide)?
(Multiple Choice)
4.9/5  (42)
(42)
How many neutrons are in an atom that has a mass number of 75 and contains 35 protons?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following units can be used to collect a sample of atoms of one element with a mass in grams equal to the atomic weight of another element?
(Multiple Choice)
4.7/5  (34)
(34)
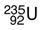 is the form of uranium used to make atomic bombs. One atom of this isotope consists of ___.
is the form of uranium used to make atomic bombs. One atom of this isotope consists of ___.
(Multiple Choice)
4.8/5  (33)
(33)
One mole of any substance will contain one Avogadro's number of atoms of that substance.
(True/False)
4.8/5  (41)
(41)
A diet is planned for a trip on a space ship and is lacking in milk, but is rich in turnips and broccoli. Such a diet could provide a sufficient amount of calcium for adults.
(True/False)
4.8/5  (39)
(39)
The symbols for all of the elements are derived from the Latin names.
(True/False)
4.8/5  (42)
(42)
One mole of an element would weigh the same as a mole of an isotope of the same element.
(True/False)
4.9/5  (30)
(30)
A mole of copper contains the same number of atoms as a mole of zinc.
(True/False)
4.8/5  (41)
(41)
If calcium carbonate found in limestone is 40.0% calcium, how many grams of calcium are in 485 g of calcium carbonate?
(Multiple Choice)
4.9/5  (37)
(37)
Refer to a periodic table and tell how many helium atoms (He)would be needed to get close to the same mass as an average oxygen atom (O).
(Multiple Choice)
4.7/5  (41)
(41)
A scanning tunneling microscope (STM)relies on a very strong light source to help see atoms.
(True/False)
4.7/5  (29)
(29)
One mole of an element would weigh the same as a mole of an isotope of the same element.
(True/False)
4.9/5  (34)
(34)
Atoms that have the same atomic number but differ by mass number are called?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 41 - 60 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)