Exam 2: Atoms and Molecules
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
Naturally occurring lithium (Li)consists of only two isotopes, Li-6 (6.02 u)and Li-7 (7.02 u), where the isotopic masses are given in parentheses. Use the periodic table and determine which isotope is present in the larger percentage in the natural element.
(Multiple Choice)
4.8/5  (33)
(33)
The most accurate way to determine atomic mass is with a mass spectrometer.
(True/False)
4.8/5  (37)
(37)
The average relative mass of an ozone molecule is 48.0 u. An ozone molecule contains only oxygen atoms. What does this molecular weight indicate about the formula of the ozone molecule?
(Multiple Choice)
4.8/5  (35)
(35)
Isotopes of the same element always have the same number of neutrons.
(True/False)
5.0/5  (37)
(37)
How many silicon atoms (Si)are contained in a 12.5 g sample of silicon?
(Multiple Choice)
4.9/5  (45)
(45)
Electrons do not make an important contribution to the mass of an atom.
(True/False)
4.8/5  (36)
(36)
You discover a new element which consists of two isotopes. The first isotope, 243X (mass = 242.45 u) comprises 40.000% of the total. The second isotope, 248X (mass = 247.11 u)accounts for the rest. What would be the average atomic mass for your new element?
(Multiple Choice)
4.7/5  (35)
(35)
The number of Cr atoms in a 26.0 g sample of chromium is x. How many atoms, expressed in terms of x, would be contained in 26.98 g of aluminum (Al)?
(Multiple Choice)
4.9/5  (41)
(41)
Approximately, how many atoms of beryllium would be required to equal the mass of 10 atoms of aluminum?
(Multiple Choice)
4.9/5  (33)
(33)
How many grams of iron (Fe)is contained in 15.8 g of Fe(OH)3?
(Multiple Choice)
4.9/5  (35)
(35)
One Avogadro's number of iron (Fe)atoms would weigh _____ .
(Multiple Choice)
4.9/5  (39)
(39)
An isotope of gallium consisting of 31 protons and 37 neutrons can be represented using the symbol shown below.
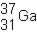
(True/False)
4.9/5  (46)
(46)
Showing 81 - 96 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)