Exam 2: Atoms and Molecules
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
How many protons are found in the nucleus of a boron-11 (11B)atom?
(Multiple Choice)
4.9/5  (35)
(35)
The first letter of the symbol for each of the elements is the first letter of its English name.
(True/False)
4.8/5  (29)
(29)
Which element is approximately 65 percent of sulfuric acid by weight?
(Multiple Choice)
4.9/5  (34)
(34)
Consider the representation shown below. It should be classified as 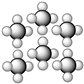
(Multiple Choice)
4.7/5  (32)
(32)
In naturally occurring samples, all elements exist as a mixture of isotopes.
(True/False)
4.9/5  (34)
(34)
Which of the following is located in the nucleus of an atom?
(Multiple Choice)
4.9/5  (36)
(36)
Using whole numbers, determine the molecular weight of calcium hydroxide, Ca(OH)2.
(Multiple Choice)
4.7/5  (38)
(38)
How many atoms are contained in a sample of krypton, Kr, that weighs 8.38 g?
(Multiple Choice)
4.8/5  (35)
(35)
How many moles of Na2Cr2O7 contain 14 moles of oxygen atoms?
(Multiple Choice)
4.9/5  (33)
(33)
What is the number of moles of water in one liter of water if one gram of water takes up one milliliter of space?
(Multiple Choice)
4.8/5  (39)
(39)
One mole of silver would contain the same number of atoms as a mole of gold.
(True/False)
4.7/5  (33)
(33)
Naturally occurring neon (Ne)has the following isotopic composition (the mass of each isotope is given in parenthesis). Calculate the atomic weight of neon in u from these data: neon-20, 90.92% (19.99 u); neon-21, 0.257% (20.99 u); neon-22, 8.82% (21.99 u)
(Multiple Choice)
4.8/5  (40)
(40)
Neutral isotopes of the same element have the same number of electrons.
(True/False)
4.9/5  (38)
(38)
A neutral isotope of an element contains 21 electrons and 24 neutrons. What is the following representation for this nucleus?
(Multiple Choice)
4.9/5  (43)
(43)
Oxalic acid is found in many plants, like spinach and black tea. What is the mass of the carbon found in one mole of oxalic acid, H2C2O4?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)