Exam 9: Acids, Bases, and Salts
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
To determine the number of equivalents of Al(NO3)3 in a 0.750 M solution, the conversion factor would be which of the following?
(Multiple Choice)
4.9/5  (35)
(35)
A 25.00 mL sample of H2SO4 acid solution requires 17.35 mL of 0.118 N base for titration. What is the normality of the acid solution?
(Multiple Choice)
4.7/5  (35)
(35)
When an inorganic acid and an inorganic base, as defined by Arrhenius, react, the products are salt and water.
(True/False)
4.9/5  (37)
(37)
What is the function of 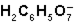 in the first ionization of citric acid?
in the first ionization of citric acid? 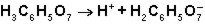
(Multiple Choice)
4.7/5  (49)
(49)
A 25.00 mL sample of hydrochloric acid solution, HCl, is titrated with 0.0512 M NaOH solution. The volume of NaOH solution required is 21.68 mL. What is the molarity of the HCl solution?
(Multiple Choice)
4.8/5  (40)
(40)
The salt of a strong acid and a weak base will give an acidic solution.
(True/False)
4.7/5  (31)
(31)
Showing 81 - 92 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)