Exam 9: Acids, Bases, and Salts
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
During a field trip to study the problem of acid rain, you test one lake that had observed a major fish kill. It was found to have a pH of 5.37. What is the [H+]?
(Multiple Choice)
5.0/5  (43)
(43)
The expression for the K a of the weak acid HF would be which of the following?
(Multiple Choice)
4.9/5  (31)
(31)
The molar concentration of H+ ions in a solution is 5.8 × 10 − 9. The pH is _____ .
(Multiple Choice)
4.7/5  (41)
(41)
Consider the following list of p K a values for a series of acids. Which is the strongest acid?
(Multiple Choice)
4.9/5  (25)
(25)
The pH of blood must remain stable between 7.35 and 7.45. One of the blood components is the bicarbonate ion, 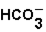 . Bicarbonate takes part in two reactions that are:
. Bicarbonate takes part in two reactions that are:
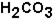 (aq)
(aq) 
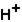 (aq)+
(aq)+ 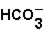 (aq)
(aq) 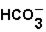

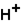 (aq)+
(aq)+ 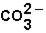 (aq)
This set of equations indicates that this system is a good candidate to act as a buffer system used to hold the pH of blood reasonably stable.
(aq)
This set of equations indicates that this system is a good candidate to act as a buffer system used to hold the pH of blood reasonably stable.
(True/False)
4.9/5  (37)
(37)
Identify the Brønsted acid(s)in the reaction. HIO3(aq)+ H2O (l)  H3O+ (aq)+ IO3- (aq)
H3O+ (aq)+ IO3- (aq)
(Multiple Choice)
4.8/5  (28)
(28)
A patient comes to you suffering from a battery acid burn (sulfuric acid). What is the best thing to use to neutralize the acid, while you continue to run cool water over the affected area?
(Multiple Choice)
4.8/5  (38)
(38)
Seageroic acid has a p K a of 8.23, whereas slabaughic acid has a p K a of 18.65. From these facts, which of the following is true?
(Multiple Choice)
5.0/5  (39)
(39)
A 10-8 M solution of HCl is prepared. It would be expected to be _____.
(Multiple Choice)
4.7/5  (36)
(36)
The dissociation reaction for the weak acid, H2PO3- would be which of the following?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following would you expect to be a weak electrolyte?
(Multiple Choice)
4.8/5  (34)
(34)
A reaction in which an acid and a base react completely, leaving only a salt and water, is referred to as a(n)
(Multiple Choice)
4.8/5  (34)
(34)
Identify all Brønsted base(s)in the reaction. N3- (aq)+ H2O (l)  HN3 (aq)+ OH- (aq)
HN3 (aq)+ OH- (aq)
(Multiple Choice)
4.9/5  (31)
(31)
When your liver detoxifies ethyl alcohol, the concentration of the hydrogen ion increases. This will result in
(Multiple Choice)
4.8/5  (35)
(35)
The pH of household ammonia is expected to be above 7 because household ammonia is NH4OH.
(True/False)
4.8/5  (45)
(45)
A solution for which [H+] = 1.0 × 10 − 3 will have a pH of _____ .
(Multiple Choice)
4.8/5  (39)
(39)
Showing 61 - 80 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)