Exam 10: Radioactivity and Nuclear Processes
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
You inject a 160 lb male patient with 1.0 ml of a saline solution containing a radioactive form of sodium. It has an activity of 5.0 × 104 dpm. After allowing sufficient time for the solution to mix, you remove a 1.0 ml sample of blood and measure its radioactivity. You discover it to have an activity of 11 dpm. What is the patient's blood volume? (Hint: this is similar to a M1V1 = M2V2 type problem.)
(Multiple Choice)
4.9/5  (36)
(36)
Which of the responses represent the missing particle in the following reaction? 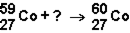
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following is a physical measurement of radioactivity?
(Multiple Choice)
4.9/5  (36)
(36)
Radioisotopes which emit alpha rays make the best diagnostic tracers.
(True/False)
4.8/5  (35)
(35)
The curie, becquerel and roentgen are all physical units of radiation.
(True/False)
4.9/5  (29)
(29)
The radioisotope radon-222 is a gas that is a health hazard because it can make its way into the house from the soil in which it is produced, be inhaled, and cause lung cancer.
(True/False)
4.9/5  (43)
(43)
There are some elements within the periodic table that do not occur in nature. One of the man-made elements is _____ .
(Multiple Choice)
4.8/5  (35)
(35)
A desirable characteristic of a radioisotope used as a tracer is that it produces a radioactive daughter.
(True/False)
4.8/5  (37)
(37)
Diagnostic tracers form cool spots when they are accumulated in diseased tissue.
(True/False)
5.0/5  (36)
(36)
How much of a fissionable material is required to have a self-sustaining reaction?
(Multiple Choice)
4.8/5  (46)
(46)
A common use of radioisotopes is to speed up chemical reactions.
(True/False)
4.8/5  (35)
(35)
Uranium-235, under the proper circumstances, will undergo nuclear decay releasing 3 high speed neutrons in the process.
(Multiple Choice)
4.9/5  (41)
(41)
A rem is a biological radiation measurement which is independent of the type of radiation.
(True/False)
4.8/5  (46)
(46)
Showing 21 - 40 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)