Exam 10: Radioactivity and Nuclear Processes
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
A good radioisotope tracer for medical use should not have the following characteristic.
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following would be associated with medical testing?
(Multiple Choice)
4.7/5  (40)
(40)
The intensity of a radiation source was measured by a Geiger-Müller counter to be 236 counts per minute when the counter was place 124 ft from the source. What will be the intensity when the counter is 248 ft from the source?
(Multiple Choice)
4.8/5  (26)
(26)
Nuclear power plants have control rods that absorb neutrons to control the reaction. What is the composition of the control rods?
(Multiple Choice)
4.9/5  (43)
(43)
Manganese can undergo several types of radioactive emission. In which type of decay does the following reaction occur? 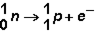
(Multiple Choice)
4.9/5  (42)
(42)
The gray is one of several units used in association with the biological impact of radiation exposure.
(True/False)
4.7/5  (27)
(27)
If you are a medical professional and have a chance to be exposed to many sources of radiation, which of the following units to measure biological radiation will most likely be used to express your level of total exposure?
(Multiple Choice)
4.8/5  (42)
(42)
Consider the figure below. Which form of emitted radiation would follow the path shown by the arrow labeled 1? 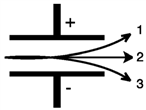
(Multiple Choice)
4.7/5  (34)
(34)
Most of the synthetic (man-made)elements are stable and have been produced in large amounts.
(True/False)
4.8/5  (37)
(37)
The following equation describes the amount of energy released in nuclear reactions.
E = mc2
(True/False)
4.7/5  (47)
(47)
Which of the following would be the correct symbol for an americium-243 nucleus using the 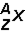 symbolism?
symbolism?
(Multiple Choice)
4.8/5  (45)
(45)
An isotope of zinc containing 36 neutrons is bombarded with and captures a proton. What isotope is produced as a product of the reaction?
(Multiple Choice)
4.8/5  (40)
(40)
A wooden box found in an Egyptian tomb could have its age determined using carbon-14.
(True/False)
4.9/5  (40)
(40)
A major problem with attempting to measure the effect of an exposure to emissions is that each different emission has a different effect on tissue due to the differences in penetration.
(True/False)
4.7/5  (40)
(40)
Which of the following would have the least penetrating power into matter?
(Multiple Choice)
4.9/5  (33)
(33)
A patient that works at a nuclear power plant comes to you complaining of nausea and fatigue. You find out that he might have been exposed to a radiation source; what would you recommend be done?
(Multiple Choice)
4.8/5  (38)
(38)
A nuclear reaction in which beta particles are emitted yields an atom that weighs 2 μ less than the starting element.
(True/False)
4.8/5  (34)
(34)
Showing 61 - 80 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)