Exam 10: Radioactivity and Nuclear Processes
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
The reasons why I-131 is not suitable for use as a tracer are the same reasons why I-131 is an appropriate choice to treat thyroid cancer.
(True/False)
4.9/5  (40)
(40)
Which of the following types of nuclear radiation consists of only positive electrons?
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following radioisotopes is not used as a diagnostic tracer?
(Multiple Choice)
4.9/5  (44)
(44)
One reason that irradiated food is not readily available in most grocery stores is that
(Multiple Choice)
4.8/5  (48)
(48)
The elements beyond uranium, the trans-uranium elements, are all synthetic.
(True/False)
4.9/5  (43)
(43)
The intensity of radiation is 100 units 15 feet from the source. The intensity would then be 300 units 5 feet from the source.
(True/False)
4.9/5  (43)
(43)
Which of the following is not attracted or repelled by electrical charges?
(Multiple Choice)
4.8/5  (34)
(34)
Consider the figure below. Which form of emitted radiation would follow the path shown by the arrow labeled 2? 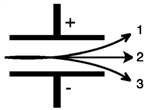
(Multiple Choice)
4.8/5  (43)
(43)
Which measure of radiation is used to account for health differences of various types of radiation?
(Multiple Choice)
4.9/5  (28)
(28)
Consider the figure below. Which form of emitted radiation would follow the path shown by the arrow labeled 3? 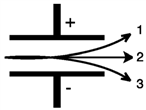
(Multiple Choice)
4.8/5  (36)
(36)
Sodium-24 has a half-life of 15.0 hours. Suppose you had a sample containing 0.010 moles of Na-24. How many hours would be required to reduce your sample to 6.25 × 10 − 4 moles?
(Multiple Choice)
4.7/5  (41)
(41)
What is one of the advantages of food that has been irradiated to kill bacteria?
(Multiple Choice)
5.0/5  (33)
(33)
Identify the mode of decay in an unstable nucleus in which an electron from outside the nucleus is drawn into the nucleus and the electron combines with a proton to form a neutron.
(Multiple Choice)
4.8/5  (36)
(36)
Tritium has a half-life of 12.5 years. If you had a sample of 8.00 grams of tritium today, how many grams of tritium would you have in 37.5 years?
(Multiple Choice)
4.8/5  (34)
(34)
The term "nuclear energy" is most closely associated with which one of the following processes?
(Multiple Choice)
4.7/5  (42)
(42)
Which technique of medical investigation presents no ionizing radiation risk to the patient?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following types of radiation is composed of particles which carry a negative charge?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 41 - 60 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)