Exam 11: Liquids and Solids
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
Which phase change listed below represents the transition from the gaseous state to the liquid state?
(Multiple Choice)
4.8/5  (37)
(37)
The property of a liquid that causes small drops to be spherical is called:
(Multiple Choice)
4.8/5  (44)
(44)
In a closest packing array of atoms, how many nearest neighbors does each atom have?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following properties of a liquid increase as the strength of intermolecular forces increases?
I. enthalpy of vaporization
II. boiling point
III. surface tension
(Multiple Choice)
4.9/5  (26)
(26)
On the basis of intermolecular forces of attraction, rank the following three compounds in terms of increasing boiling point. CH3CH2CH2CH2CH3 (Pentane), CHCl3 (Chloroform) and H2O (Water)
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following phase changes is an exothermic event?
I. Sublimation
II. Condensing
III. Melting
(Multiple Choice)
4.9/5  (31)
(31)
Exhibit 11-4 Consider Aluminum metal that crystallizes in a face centered cubic cell to answer the following problem(s). The atomic radius of an aluminum atom is 1.43 . One face of this unit cell is shown in the diagram below. 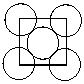 -Refer to Exhibit 11-4. What is the volume of this unit cell?
-Refer to Exhibit 11-4. What is the volume of this unit cell?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following phase changes listed below would be considered endothermic ?
I. Sublimation
II. Deposition
III. Condensation
(Multiple Choice)
4.7/5  (42)
(42)
What type of crystal forces hold the units together in solid sodium iodide?
(Multiple Choice)
4.8/5  (36)
(36)
How many unit cells share an atom at the corner of a cubic unit cell?
(Multiple Choice)
4.8/5  (38)
(38)
Cesium chloride crystallizes in a unit cell with the Cesium ions in a primitive cubic unit cell arrangement (at the corners). Given that the formula for cesium chloride is CsCl, where are the chloride ions expected to reside in the unit cell?
(Multiple Choice)
4.9/5  (26)
(26)
Three molecules are listed below. Will hydrogen bonding be important for any of them?
Pick the correct answer.
I. HBr
II. CH3OH
III. NH3
(Multiple Choice)
4.8/5  (32)
(32)
Exhibit 11-1 The heating curve below is needed for the following question(s). 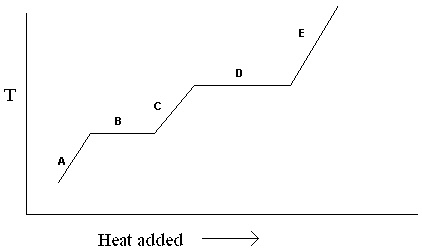 Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where only solid is present?
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where only solid is present?
(Multiple Choice)
4.9/5  (36)
(36)
In a simple cubic unit cell comprised of uniform size spheres, what is the coordination number of each sphere?
(Multiple Choice)
4.9/5  (38)
(38)
Given the three statements below, pick the best answer.
I. The normal boiling point of a liquid is the temperature at which its vapor pressure is 1 atm.
II. Water always boils at 100 ° C.
III. The vapor pressure of a liquid increases with increasing temperature.
(Multiple Choice)
4.9/5  (42)
(42)
Exhibit 11-1 The heating curve below is needed for the following question(s). 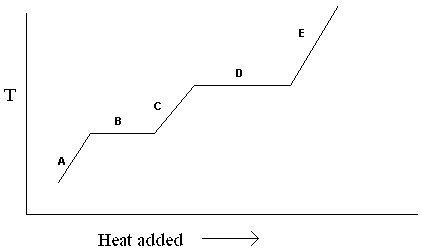 Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment that corresponds to the heat of vaporization?
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment that corresponds to the heat of vaporization?
(Multiple Choice)
4.8/5  (33)
(33)
Arrange the following elements in order of increasing boiling point. Argon, Helium, Neon
(Multiple Choice)
4.9/5  (39)
(39)
At room temperature chlorine is a gas, bromine is a liquid, and iodine is a solid. All three exist as diatomic molecules at this temperature. The intermolecular attractions in these substances increase in the order:
(Multiple Choice)
4.7/5  (39)
(39)
Formaldehyde is a colorless gas but when dissolved in water can be used in embalming fluids. Which intermolecular force(s) is(are) present among formaldehyde molecules shown below?
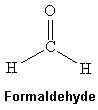
(Multiple Choice)
4.9/5  (45)
(45)
Showing 21 - 40 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)