Exam 11: Liquids and Solids
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
Which of the following forces between atoms or ions or molecules is the strongest on a per mole basis?
(Multiple Choice)
4.8/5  (46)
(46)
Exhibit 11-2 The phase diagram below is needed for the following question(s). 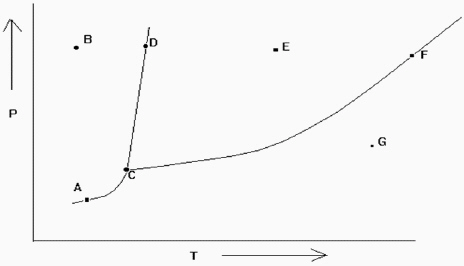 Refer to Exhibit 11-2. Which point denotes the place where all the three phases are in equilibrium at the same time?
Refer to Exhibit 11-2. Which point denotes the place where all the three phases are in equilibrium at the same time?
(Multiple Choice)
4.9/5  (35)
(35)
Which intermolecular forces of attraction represents an interaction between two molecules resulting from opposites charges (partial positive and partial negative interactions)?
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
(Multiple Choice)
4.9/5  (26)
(26)
Which crystal packing structure listed below provides an "ABA" closest packing arrangement for the different layers of metal spheres?
(Multiple Choice)
4.8/5  (37)
(37)
The property of a liquid that measures its resistance to flowing is:
(Multiple Choice)
4.8/5  (39)
(39)
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 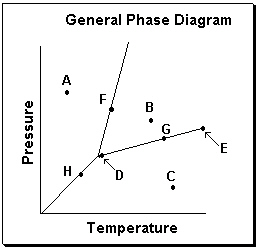 -Refer to Exhibit 11-3. Which transition is related to sublimation in the phase diagram above?
-Refer to Exhibit 11-3. Which transition is related to sublimation in the phase diagram above?
(Multiple Choice)
4.8/5  (31)
(31)
Calcium fluoride, CaF2, has a unit cell with the Ca2+ ions in a face-centered cubic arrangement. How many fluoride ions are present in the unit cell?
(Multiple Choice)
4.9/5  (37)
(37)
Exhibit 11-2 The phase diagram below is needed for the following question(s). 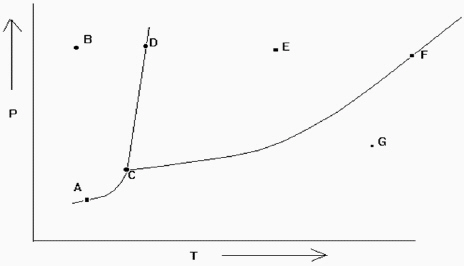 Refer to Exhibit 11-2. If one is at position F and the pressure is increased, which statement below is true?
Refer to Exhibit 11-2. If one is at position F and the pressure is increased, which statement below is true?
(Multiple Choice)
4.8/5  (42)
(42)
The basic repeating three-dimensional pattern of a solid is known as the:
(Multiple Choice)
4.8/5  (43)
(43)
Exhibit 11-4 Consider Aluminum metal that crystallizes in a face centered cubic cell to answer the following problem(s). The atomic radius of an aluminum atom is 1.43 . One face of this unit cell is shown in the diagram below. 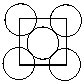 -Refer to Exhibit 11-4. How many aluminum atoms are present in a single face centered unit cell?
-Refer to Exhibit 11-4. How many aluminum atoms are present in a single face centered unit cell?
(Multiple Choice)
4.9/5  (38)
(38)
At what angle are x-rays with a wavelength of 175 pm diffracted by layers of atoms that are 353 pm apart?
(Assume n = 1)
(Multiple Choice)
4.8/5  (34)
(34)
Exhibit 11-2 The phase diagram below is needed for the following question(s). 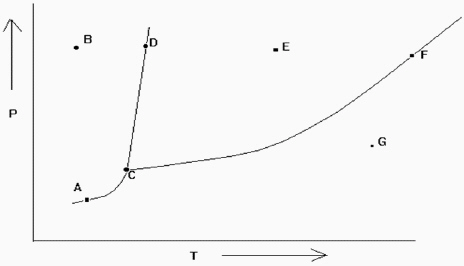 Refer to Exhibit 11-2. If one is at position A and the pressure is increased, which statement below is true?
Refer to Exhibit 11-2. If one is at position A and the pressure is increased, which statement below is true?
(Multiple Choice)
4.9/5  (40)
(40)
A crystal diffracts x-rays (£ = 154 pm) at an angle of 16.4 ° . What is the spacing between the layers of atoms that produced this diffraction?
(Assume the order of diffraction is one.)
(Multiple Choice)
4.9/5  (47)
(47)
For most substances, the enthalpy of the phase changes, D H sublimation, D H fusion, and D H vaporization, increase in the order:
(Multiple Choice)
4.7/5  (30)
(30)
On the basis of intermolecular forces of attraction, rank the following three compounds in terms of increasing boiling point . NH3 (Ammonia), N2 (Nitrogen) and HCl (Hydrogen Chloride)
(Multiple Choice)
4.7/5  (28)
(28)
Which crystal packing structure listed below provides an "ABC" closest packing arrangement for the different layers of metal spheres?
(Multiple Choice)
5.0/5  (41)
(41)
Which unit cell(s) provide(s) a coordination number of 12 for each atom in the lattice?
(Multiple Choice)
4.8/5  (37)
(37)
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 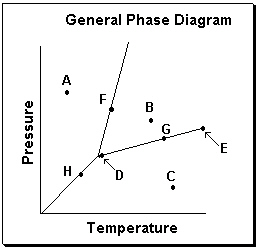 -Refer to Exhibit 11-3. What temperature-pressure point on the General Phase Diagram above represents the Critical Point ?
-Refer to Exhibit 11-3. What temperature-pressure point on the General Phase Diagram above represents the Critical Point ?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following forces between atoms or ions or molecules is the strongest?
(Multiple Choice)
4.8/5  (31)
(31)
Showing 81 - 100 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)