Exam 11: Liquids and Solids
Exam 1: Introduction to Chemistry95 Questions
Exam 2: Atoms, Molecules, and Ions132 Questions
Exam 3: Equations, the Mole, and Chemical Formulas186 Questions
Exam 4: Chemical Reactions in Solution107 Questions
Exam 5: Thermochemistry78 Questions
Exam 6: The Gaseous State140 Questions
Exam 7: Electronic Structure86 Questions
Exam 8: The Periodic Table: Structure and Trends90 Questions
Exam 9: Chemical Bonds119 Questions
Exam 10: Molecular Structure and Bonding Theories133 Questions
Exam 11: Liquids and Solids100 Questions
Exam 12: Solutions199 Questions
Exam 13: Chemical Kinetics148 Questions
Exam 14: Chemical Equilibrium212 Questions
Exam 15: Solutions of Acids and Bases179 Questions
Exam 16: Reactions Between Acids and Bases98 Questions
Exam 17: Chemical Thermodynamics106 Questions
Exam 18: Electrochemistry112 Questions
Exam 19: Transition Metals, Coordination Chemistry and Metallurgy73 Questions
Exam 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases41 Questions
Exam 21: Nuclear Chemistry89 Questions
Exam 22: Organic Chemistry and Biochemistry175 Questions
Select questions type
In a body-centered cubic array of uniform size spheres, the coordination number of each sphere is:
(Multiple Choice)
4.9/5  (31)
(31)
A metal that crystallizes in a face-centered cubic array of atoms has a radius of 124 pm. What is the length of the edge for the unit cell, in pm?
(Multiple Choice)
4.7/5  (37)
(37)
Exhibit 11-3 Consider the General Phase Diagram shown below to answer the following problem(s). 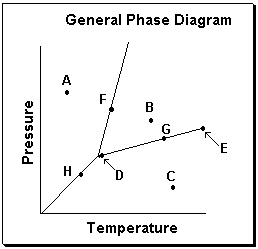 -Refer to Exhibit 11-3. What phase transition would occur upon moving from point B to point A on the phase diagram above?
-Refer to Exhibit 11-3. What phase transition would occur upon moving from point B to point A on the phase diagram above?
(Multiple Choice)
4.9/5  (35)
(35)
Which molecule listed below would have its intermolecular forces dominated by dispersion forces?
(Multiple Choice)
4.7/5  (43)
(43)
A crystal diffracts x-rays (£ = 154 pm) at an angle of 9.8 ° . What is the spacing between the layers of atoms that produced this diffraction?
(Assume the order of diffraction is one.)
(Multiple Choice)
4.8/5  (29)
(29)
Aluminum metal crystallizes in a face centered cubic cell . One face of this unit cell is shown in the diagram that follows. The length along one edge equals 4.04 . 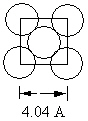 What is the atomic radius of an aluminum atom?
What is the atomic radius of an aluminum atom?
(Multiple Choice)
4.8/5  (38)
(38)
A metallic element crystallizes in a face-centered cubic array of atoms, for which the length of the cube edge is 351 pm. What is the atomic radius of the metal, in pm?
(Multiple Choice)
4.8/5  (31)
(31)
Exhibit 11-2 The phase diagram below is needed for the following question(s). 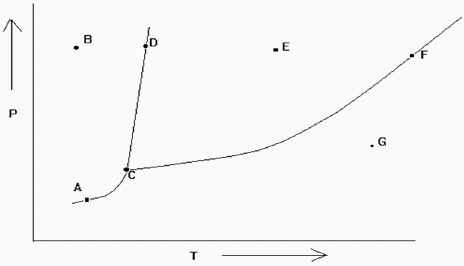 Refer to Exhibit 11-2. If the temperature and pressure are such that the substance is at point D in the diagram, a slight increase in temperature would cause what type of change?
Refer to Exhibit 11-2. If the temperature and pressure are such that the substance is at point D in the diagram, a slight increase in temperature would cause what type of change?
(Multiple Choice)
4.8/5  (39)
(39)
Based upon intermolecular forces of attraction, which is the correct order for increasing boiling points among the following three molecules?
N2, H2, and NH3
(Multiple Choice)
4.9/5  (41)
(41)
Exhibit 11-1 The heating curve below is needed for the following question(s). 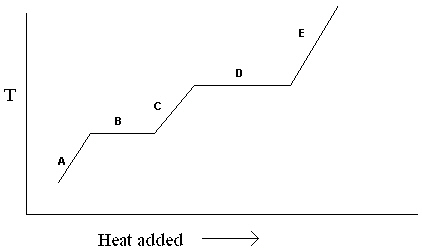 Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where liquid and gas may be present?
Refer to Exhibit 11-1. For the heating curve shown, which letter labels the line segment where liquid and gas may be present?
(Multiple Choice)
4.9/5  (32)
(32)
What intermolecular force(s) of attraction is(are) present between two molecules of ethane shown below?
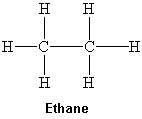 I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following phase transitions are considered exothermic ?
I. Freezing
II. Condensation
III. Deposition
(Multiple Choice)
4.8/5  (34)
(34)
In a cubic closest packing array of identical atoms, the coordination number of each atom is:
(Multiple Choice)
4.9/5  (39)
(39)
The term that best explains why water rises up a thin glass tube is:
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following would have a boiling point lower than SiCl4?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the three states of matter have much greater kinetic energy than their forces of attraction?
I. Solids
II. Liquids
III. Gases
(Multiple Choice)
4.8/5  (25)
(25)
Given the three statements below, pick the best answer.
I. Dipole-dipole interactions should contribute to the intermolecular bonding forces of FBr.
II. London forces (instantaneous dipole) are the only intermolecular forces acting between H2 molecules.
III. London forces should be greater for I2 than Br2 because I2 is more polarizable.
(Multiple Choice)
4.7/5  (42)
(42)
Iron crystallizes in a body-centered cubic array of atoms. What is the number of atoms per unit cell for this metal?
(Multiple Choice)
4.9/5  (32)
(32)
What intermolecular force(s) is(are) present among molecules of HBr?
I. London dispersion
II. Dipole-dipole
III. Hydrogen bonding
(Multiple Choice)
4.8/5  (40)
(40)
Showing 61 - 80 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)