Exam 9: Hydrocarbons: an Introduction to Organic Molecules
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
Which of the following is(are) constitutional isomer(s) of hexane?
(Multiple Choice)
4.8/5  (32)
(32)
What is line bond formula for the following full structural formula? 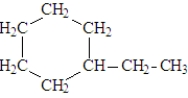
(Multiple Choice)
4.9/5  (35)
(35)
Consider the following structure. 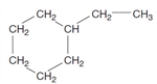 This substance would be classified as a(n)
This substance would be classified as a(n)
(Multiple Choice)
4.8/5  (31)
(31)
Consider the formula given below. C4H8 This following represents the molecular formula of an
(Multiple Choice)
4.9/5  (33)
(33)
Consider the following structures. 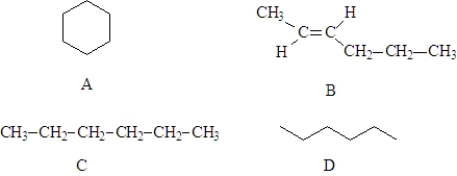 Fill in the blanks with the appropriate letters (A, B, C, or D).
-Structure A is a constitutional isomer of Structure _____________________.
Fill in the blanks with the appropriate letters (A, B, C, or D).
-Structure A is a constitutional isomer of Structure _____________________.
(Short Answer)
4.8/5  (33)
(33)
Examine the following structure. 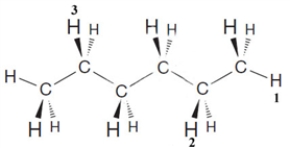 Complete the following statements about this structure.
-The IUPAC name for this substance is____________.
Complete the following statements about this structure.
-The IUPAC name for this substance is____________.
(Short Answer)
4.7/5  (37)
(37)
During the complete combustion of benzene, if 9.55 g of benzene were consumed what mass of oxygen in grams would be needed?
(Short Answer)
4.8/5  (30)
(30)
Examine the following structures. 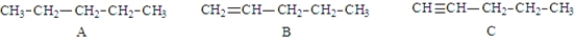 Fill in the blanks with the appropriate letter (A, B, C, ....).
-Structure D is classified as a(n) _________________________.
Fill in the blanks with the appropriate letter (A, B, C, ....).
-Structure D is classified as a(n) _________________________.
(Short Answer)
4.9/5  (33)
(33)
How many hydrogen atoms would be needed to complete the following structure? 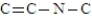
(Multiple Choice)
4.9/5  (40)
(40)
Examine the following structure.  Complete the following statements about this structure.
-If the hydrogen atom numbered "1" replaced by a methyl group, the IUPAC name of the new compound would be ____________.
Complete the following statements about this structure.
-If the hydrogen atom numbered "1" replaced by a methyl group, the IUPAC name of the new compound would be ____________.
(Short Answer)
4.9/5  (35)
(35)
What must be the name for the missing hydrocarbon in the following combustion reaction? _______ + 3 O2 → 2 CO2 + 2 H2O
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following hydrocarbons is most likely to be a gas at room temperature?
(Multiple Choice)
4.9/5  (37)
(37)
The following compound contains four tetrahedrally arranged atoms. 
(True/False)
4.9/5  (34)
(34)
Consider the compounds given in the choices. Which would have the lowest melting point?
(Multiple Choice)
4.8/5  (36)
(36)
Carbon atoms involved in double bonds exhibit a trigonal planar arrangement.
(True/False)
4.9/5  (32)
(32)
Showing 21 - 40 of 74
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)