Exam 9: Hydrocarbons: an Introduction to Organic Molecules
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
The two compounds shown below have the same molecular formula. CH3-CH2-O-CH2-CH2-CH3 CH3-CH2-CH2-CH2-CH2-OH
(True/False)
4.8/5  (32)
(32)
Consider the following molecule of the birth control drug known as RU 486. 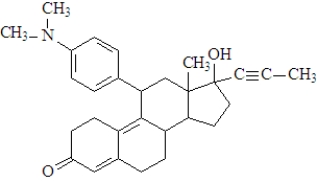 Fill in the blanks with the appropriate term from the list below.
alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
-The functional group enclosed in the triangle is a(n)_____________________.
Fill in the blanks with the appropriate term from the list below.
alkane
cycloalkane
alkene
cycloalkene
alkyne
aromatic
haloalkane
-The functional group enclosed in the triangle is a(n)_____________________. 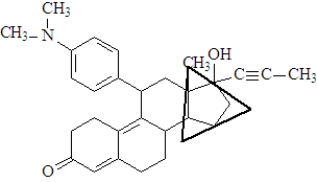
(Short Answer)
4.9/5  (34)
(34)
Examine the following structures. 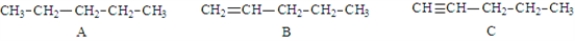 Fill in the blanks with the appropriate letter (A, B, C, ....).
-Structure ____________________ will have the lowest melting point.
Fill in the blanks with the appropriate letter (A, B, C, ....).
-Structure ____________________ will have the lowest melting point.
(Short Answer)
4.9/5  (37)
(37)
Examine the following structures. 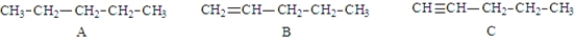 Fill in the blanks with the appropriate letter (A, B, C, ....).
-Structure B is classified as a(n) _________________________.
Fill in the blanks with the appropriate letter (A, B, C, ....).
-Structure B is classified as a(n) _________________________.
(Short Answer)
4.8/5  (35)
(35)
Using molecular formula, write the balance equation for the complete combustion of benzene.
(Essay)
4.9/5  (38)
(38)
Examine the following structures. 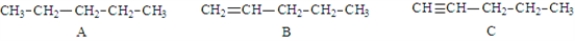 Fill in the blanks with the appropriate letter (A, B, C, ....).
-The balanced equation for the combustion of structure _______________________ would required the largest coefficient for O2.
Fill in the blanks with the appropriate letter (A, B, C, ....).
-The balanced equation for the combustion of structure _______________________ would required the largest coefficient for O2.
(Short Answer)
4.8/5  (44)
(44)
Consider the following structure. 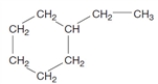 What is the relationship to the name given below? 2,3-dimethylhexane
What is the relationship to the name given below? 2,3-dimethylhexane
(Multiple Choice)
4.8/5  (29)
(29)
Given the structure below, what is the IUPAC name for the compound? 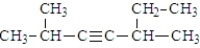
(Multiple Choice)
4.9/5  (37)
(37)
The two compounds shown below would be expected to exhibit similiar properties. CH3-CH2-O-CH2-CH2-CH3 CH3-CH2-CH2-CH2-CH2-OH
(True/False)
4.8/5  (36)
(36)
Which of the following is could not be used to represent the same compound?
(Multiple Choice)
4.8/5  (30)
(30)
What is the name of a branch on the principal carbon chain that contains three carbon atoms?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 41 - 60 of 74
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)