Exam 7: Acids and Bases
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
Which of the following substances can react with both HCl and NaOH?.
(Multiple Choice)
4.8/5  (30)
(30)
A solution composed of HCl and NaCl would produce an effective buffer.
(True/False)
4.7/5  (36)
(36)
When NaOH reacts with HCl, the net ionic equation for the reaction is:
(Multiple Choice)
4.9/5  (41)
(41)
Consider the following reaction. 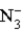 + H2O
+ H2O 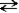 HN3 + OH− This reaction indicates that:
HN3 + OH− This reaction indicates that:
(Multiple Choice)
4.9/5  (26)
(26)
The buffer solution in the flask was prepared using one of the substances shown below. citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below,  complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
-If HNO were added to the buffer solution the pH of the solution would __________.
complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
-If HNO were added to the buffer solution the pH of the solution would __________.
(Short Answer)
4.8/5  (35)
(35)
Which of the following will determine the pH of a buffer made using acetic acid (HC2H3O2) and sodium acetate (NaC2H3O2)?
(Multiple Choice)
4.7/5  (29)
(29)
A solution is 0.10 M in ascorbic acid (Vitamin C), H2C6H6O6, and 0.10 M in sodium ascorbate, NaHC6H6O6. When base is added to the solution, H2C6H6O6 neutralizes the added base.
(True/False)
4.9/5  (37)
(37)
When carbonated beverages are bottled or canned, the partial pressure of carbon dioxide above this liquid is maintained at a high pressure. This procedure cause the pH of the beverage to
(Multiple Choice)
4.9/5  (47)
(47)
Write the equation for the ionization of weak base hydrazine, N2H4, in water.
(Essay)
4.9/5  (39)
(39)
Excess phosphorus is excreted by the kidneys. What effect does this have on the pH of blood plasma?
(Multiple Choice)
4.7/5  (40)
(40)
In a solution of phosphoric acid all of the following species are present. Which is the least abundant?
PO43-
H2PO4-
HPO42-
(Short Answer)
4.8/5  (32)
(32)
Very rapid breathing can lead to a condition know as respiratory acidosis.
(True/False)
4.8/5  (48)
(48)
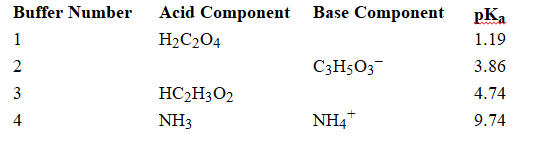 Using the above table, fill in the blank with the appropriate integer (1 ,2, 3,...) indicating the buffer and/or the letter indicating the formula below.
-Buffer ______________________ would be the most effective buffer in basic solutions.
Using the above table, fill in the blank with the appropriate integer (1 ,2, 3,...) indicating the buffer and/or the letter indicating the formula below.
-Buffer ______________________ would be the most effective buffer in basic solutions.
(Short Answer)
4.9/5  (42)
(42)
The pKa of lactic acid is 3.86, an equimolar solution of lactic acid and potassium lactate will have a pH of 7.72.
(True/False)
4.9/5  (37)
(37)
Consider the following three buffer reactions. Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1, 2, or 3) to indicate the buffer system described.
-Buffer______________________can have a variable pKa value.
(Short Answer)
4.8/5  (30)
(30)
Which of the following pH's corresponds to a highly acidic solution?
(Multiple Choice)
4.9/5  (46)
(46)
Below is the structure for the amino acid alanine. 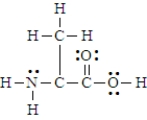 Alanine can undergo an internal acid-base reaction.
Alanine can undergo an internal acid-base reaction. 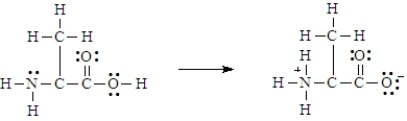 In this reaction, the -NH2 group functions as a base.
In this reaction, the -NH2 group functions as a base.
(True/False)
4.8/5  (40)
(40)
The range of breaths per minute, also known as respiratory rate, for a normal, healthy adult is 12-20 breaths per minute. A breathing rate of higher than this is termed hyperventilation.
(True/False)
4.9/5  (33)
(33)
Oxalic acid (H2C2O4) is weak acid found in the leaves of rhubarb. Which of the following solutions will have the highest pH?
(Multiple Choice)
4.9/5  (38)
(38)
The following reaction represents the self-ionization of ammonia (NH3). NH3(aq) + NH3(aq) 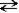 NH2-(aq) + NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In this reaction, the conjugate base of ammonia is the _______________________ ion.
NH2-(aq) + NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In this reaction, the conjugate base of ammonia is the _______________________ ion.
(Short Answer)
5.0/5  (36)
(36)
Showing 41 - 60 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)