Exam 7: Acids and Bases
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
Joshua has had high blood pressure for a number of years. At Joshua's last checkup, his doctor orders tests to measure the amount of creatinine in Joshua's blood and urine. Creatinine is a compound that forms whenever muscles break down creatine, a chemical that helps supply energy for muscle contractions.
a.) Does everyone have creatine in their circulating blood?
b.) Does everyone secrete creatinine in their urine? Why or why not?
(Essay)
4.7/5  (45)
(45)
The following is an accurate description of the ionization of HBr in water. 
(True/False)
4.9/5  (36)
(36)
Which of the following is excreted by the kidneys to regulate the effect of excess protein in the diet?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following is the conjugate acid of ammonia, NH3?
(Multiple Choice)
4.9/5  (44)
(44)
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants: Products: 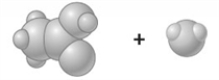
 If the composition of the equilibrium mixture is as represented below
If the composition of the equilibrium mixture is as represented below 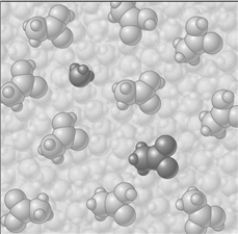 CH3COOH is classified as
CH3COOH is classified as
(Multiple Choice)
4.9/5  (45)
(45)
The buffer solution in the flask was prepared using one of the substances shown below. citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below,  complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
-The buffer solution in the beaker could be prepared using__________.
complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
-The buffer solution in the beaker could be prepared using__________.
(Short Answer)
4.8/5  (39)
(39)
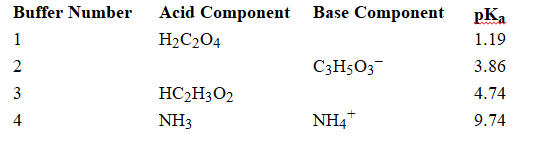 Using the above table, fill in the blank with the appropriate integer (1 ,2, 3,...) indicating the buffer and/or the letter indicating the formula below.
-In Buffer 2,_________________________ would react with added acid.
Using the above table, fill in the blank with the appropriate integer (1 ,2, 3,...) indicating the buffer and/or the letter indicating the formula below.
-In Buffer 2,_________________________ would react with added acid.
(Short Answer)
4.8/5  (35)
(35)
For a healthy individual, the pH of the blood in the veins is lower than in the arteries.
(True/False)
4.7/5  (31)
(31)
Metabolic acidosis resulting from vigorous exercise is associated with increased production of which of the following?
(Multiple Choice)
4.9/5  (35)
(35)
An aqueous solution contains a [H3O+] that is 1.0 × 10-3 M. What is the [OH-] of this solution?
(Multiple Choice)
4.9/5  (40)
(40)
Ascorbic acid (Vitamin C), H2C6H6O6, has a pKa of 4.10. A solution is 0.10 M in ascorbic acid and 0.20 M in sodium ascorbate, NaHC6H6O6. What is the approximate pH of the solution?
(Multiple Choice)
4.8/5  (40)
(40)
The following reaction represents the self-ionization of ammonia (NH3). NH3(aq) + NH3(aq) 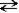 NH2-(aq) + NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In the reverse reaction, __________________________ion functions as an acid.
NH2-(aq) + NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In the reverse reaction, __________________________ion functions as an acid.
(Short Answer)
4.8/5  (40)
(40)
Adipic acid has the formula given below. 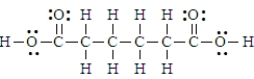 Thus substance would be classified as amphiprotic.
Thus substance would be classified as amphiprotic.
(True/False)
5.0/5  (38)
(38)
Both the kidneys and the carbon dioxide-carbonic acid cycle help regulate blood pH.
(True/False)
4.8/5  (40)
(40)
Consider the following three buffer reactions. Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1, 2, or 3) to indicate the buffer system described.
-Buffer__________________is found in both plasma and red blood cells.
(Short Answer)
4.9/5  (39)
(39)
Showing 21 - 40 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)