Exam 5: Solution Concentration
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
Consider the two containers shown below separated by a seminpermeable membrane. When allowed to stand overnight,  water will have moved from the right-hand container to the left hand container..
water will have moved from the right-hand container to the left hand container..
(True/False)
4.8/5  (45)
(45)
A solution is made by dissolving 22.5 mL of oil in enough gasoline to give 60.5 mL of solution. What is the % (v/v) of oil in the solution?
(Multiple Choice)
4.8/5  (37)
(37)
An oral rehydration solution contains 30 mEq/L of citrate3-, what is the molarity of citrate in this solution?
(Multiple Choice)
4.8/5  (40)
(40)
The total molarity of an intravenous solution is given on the label as 151 mEq/L. The osmolarity of this solution is also 151 mEq/L.
(True/False)
4.9/5  (50)
(50)
Based on the following graph, approximately what minimum temperature would be needed to dissolved 20 g of this solute in 1.00 L of water? 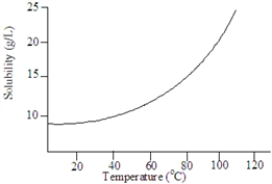
(Multiple Choice)
4.8/5  (31)
(31)
The following equation can be used when C represents either a M or % (w/v) concentration. 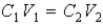
(True/False)
4.9/5  (36)
(36)
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right, left, or no movement.
-Upon standing the water molecules will move in which direction?
Answer the following questions as appropriate with: right, left, or no movement.
-Upon standing the water molecules will move in which direction?
(Short Answer)
4.8/5  (40)
(40)
Consider the two containers shown below separated by a seminpermeable membrane. When allowed to stand overnight,  there will be no change in the liquid levels.
there will be no change in the liquid levels.
(True/False)
4.9/5  (30)
(30)
Which of the following is(are) an example(s) of a hypertonic intravenous solution? (NS represents normal saline.)
(Multiple Choice)
4.9/5  (32)
(32)
How would the following solution be classified? 0.05 M in KCl and 0.14 M in glucose
(Multiple Choice)
4.7/5  (26)
(26)
Consider two solutions, A and B, separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 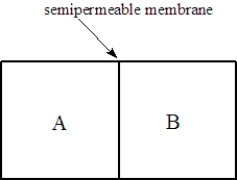 Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.10 M in lactose and 0.050 M in urea. Solution B is 0.15 M in KCl. Water will flow to the ______________________compartment.
Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.10 M in lactose and 0.050 M in urea. Solution B is 0.15 M in KCl. Water will flow to the ______________________compartment.
(Short Answer)
4.7/5  (39)
(39)
Consider two solutions, A and B, separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
-If the seminpermeable membrane has pores too small to allow glucose to pass through, ______________will not occur.
Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
-If the seminpermeable membrane has pores too small to allow glucose to pass through, ______________will not occur.
(Short Answer)
5.0/5  (41)
(41)
In cases of cerebral endema, a hypertonic solution is administered. The goal of giving the patient the hypertonic solution would be to pull fluid from the cells through the process of osmosis.
(True/False)
4.8/5  (42)
(42)
A solution contains 5.75 mg of magnesium ions in 332 mL of solution. What is the concentration of the solution in mEq/L?
(Multiple Choice)
4.9/5  (43)
(43)
If 1.00 mol of each of the following solutes is dissolved in 2.00 L of an aqueous solution, which solution contains the largest number of solute particles?
(Multiple Choice)
4.9/5  (38)
(38)
There is a 9 M aqueous HCl solution in the stock room, but a 5 M solution is required for an experiment. Doubling the volume of the 9 M sample with water will produce the 5 M solution.
(True/False)
4.9/5  (41)
(41)
The concentration of cholesterol in plasma was determined to be 215 mg/dL. The mass of cholesterol in 59.1 mL of this plasma is 127 mg.
(True/False)
4.8/5  (43)
(43)
Showing 21 - 40 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)