Exam 1: Measurements in Science and Medicine
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
Aluminum has a density of 2.70 g/ mL. What volume is occupied by a block of aluminum that weighs 4.32 kg?
(Multiple Choice)
4.9/5  (31)
(31)
How many minutes are in a 30 day month? [Assume exactly 24 hours in a day]
(Multiple Choice)
4.8/5  (30)
(30)
An angiotensin converting enzyme (ACE) inhibitor, from the dicarboxylate-containing agent group, Lisinopril, is ordered as an oral anti-hypertensive (blood pressure lowering) medication. The order reads, Lisinopril 50 mg po (by mouth) qd (each day). The pharmacy supplied Lisinopril 20 mg tablets. How many tablets would be needed each day?
(Short Answer)
4.7/5  (38)
(38)
A 20.00 mL urine sample of a patient has a mass of 20.70
g. This patient is most likely drinking very large amounts of water.
(True/False)
4.7/5  (38)
(38)
Consider the image showing a sample of modeling clay. 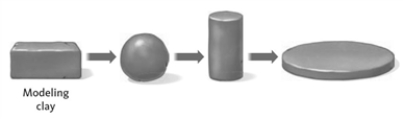 The density of the clay remains constant through the changes shown.
The density of the clay remains constant through the changes shown.
(True/False)
4.9/5  (36)
(36)
The thermostat on an incubator reads 65°C. What is this temperature on the Kelvin scale?
(Multiple Choice)
4.9/5  (24)
(24)
What temperature on the Celsius is the same as normal body temperature 98.6°F?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is true of the relationship between density and specific gravity?
(Multiple Choice)
4.7/5  (40)
(40)
The normal range (adult) for specific gravity of urine is 1.020 - 1.028 g/mL
(True/False)
4.8/5  (36)
(36)
Which of the following is not a base unit of the metric system?
(Multiple Choice)
4.8/5  (33)
(33)
To convert feet to inches, you should multiply by the factor shown below. 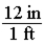
(True/False)
4.9/5  (35)
(35)
At what temperature do the temperatures on the Celsius and Kelvin scales have the same numerical value?
(Multiple Choice)
4.9/5  (32)
(32)
In the health sciences, learning and understanding how to accurately convert chemical quantities is of utmost importance, as it keeps your patients and your practice safe. At home medicines are sometimes dispensed by the teaspoon (tsp) or tablespoon (tbsp). If there are 3 tsp in 1 tbsp and 1 tbsp is equal to 15 mL, how many milliliters are 2.0 tsp?
(Multiple Choice)
4.8/5  (31)
(31)
Consider the image shown below.  Which of the following is an appropriate unit to place on this measurement?
Which of the following is an appropriate unit to place on this measurement?
(Multiple Choice)
4.9/5  (36)
(36)
Consider the image below.  The smallest division on the ruler is a cm.
The smallest division on the ruler is a cm.
(True/False)
4.8/5  (39)
(39)
The following questions refer to the plastic box shown below. 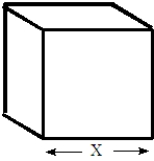 Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
dm
L
μL
g/mL
kg
μm
km
-If the box were placed on a balance, a unit that might appear on the balance read-out would be _______________________.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
dm
L
μL
g/mL
kg
μm
km
-If the box were placed on a balance, a unit that might appear on the balance read-out would be _______________________.
(Short Answer)
4.9/5  (34)
(34)
Fill in the blanks, respectively, with a letter (A or B) to represent the balance and more or less to describe the precision. The density of a metal block was determined based on mass measurements using two different balances. The results are shown below. 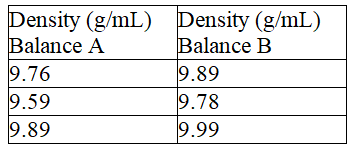 The density determined using balance ___________________ is ____________________precise.
The density determined using balance ___________________ is ____________________precise.
(Short Answer)
4.9/5  (41)
(41)
The boiling point of liquid nitrogen is 77 K. What is this temperature on the Celsius scale?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 21 - 40 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)