Exam 2: Molecular Interactions
Exam 1: Introduction to Physiology69 Questions
Exam 2: Molecular Interactions149 Questions
Exam 3: Compartmentation: Cells and Tissues160 Questions
Exam 4: Energy and Cellular Metabolism147 Questions
Exam 5: Membrane Dynamics144 Questions
Exam 6: Communication, Integration, and Homeostasis82 Questions
Exam 7: Introduction to the Endocrine System76 Questions
Exam 8: Neurons: Cellular and Network Properties229 Questions
Exam 9: The Central Nervous System107 Questions
Exam 10: Sensory Physiology175 Questions
Exam 11: Efferent Division: Autonomic and Somatic Motor Control78 Questions
Exam 12: Muscles106 Questions
Exam 13: Integrative Physiology I: Control of Body Movement76 Questions
Exam 14: Cardiovascular Physiology191 Questions
Exam 15: Blood Flow and the Control of Blood Pressure125 Questions
Exam 16: Blood114 Questions
Exam 17: Mechanics of Breathing118 Questions
Exam 18: Gas Exchange and Transport87 Questions
Exam 19: The Kidneys76 Questions
Exam 20: Integrative Physiology II: Fluid and Electrolyte Balance83 Questions
Exam 21: The Digestive System140 Questions
Exam 22: Metabolism and Energy Balance133 Questions
Exam 23: Endocrine Control of Growth and Metabolism100 Questions
Exam 24: The Immune System120 Questions
Exam 25: Integrative Physiology III: Exercise62 Questions
Exam 26: Reproduction and Development124 Questions
Select questions type
Isotopes of the same element differ by having different numbers of
(Multiple Choice)
4.8/5  (30)
(30)
What is the pH of a 0.005 M HCl solution? Assume complete dissociation.
(Essay)
4.8/5  (33)
(33)
Write the chemical formula for the molecule drawn below.Which class of organic molecule does it belong to? Is it most likely polar or nonpolar?
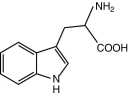
(Essay)
4.8/5  (41)
(41)
When two or more atoms are chemically linked, the smallest unit of the resulting material is referred to as a(n)
(Multiple Choice)
4.9/5  (39)
(39)
A molecule of sucrose has a molecular weight of 342 Daltons.How many grams of sucrose would be required to make one liter of a 2.5 Molar solution of sucrose?
(Essay)
4.9/5  (29)
(29)
Which of the following elements combine to form nonpolar covalent bonds?
(Multiple Choice)
4.8/5  (28)
(28)
What makes fats solid at room temperature? The more likely a fat is to be solid at room temperature the more it potentially can contribute to cardiovascular disease.With this in mind which fats will be the most dangerous?
(Essay)
4.8/5  (31)
(31)
Lipids are hydrophobic, and do not usually dissolve in water.Because blood is water-based, the lipid cholesterol is combined with ________ so that it can be transported by blood.
(Multiple Choice)
4.8/5  (43)
(43)
Each of the following is a function of proteins EXCEPT one.Identify the exception.
(Multiple Choice)
4.8/5  (36)
(36)
Ionic bonds are considered to be strong chemical bonds.Yet, ions dissociate in water.Explain this apparent contradiction.
(Essay)
4.8/5  (40)
(40)
Nucleotides participate in which of the following functions?
(Multiple Choice)
4.9/5  (41)
(41)
Lipids are considered hydrophobic because they easily dissolve in water.If True what allows them to dissolve in water or if not, what is it about their molecular structure that makes them less likely to dissolve in water?
(True/False)
4.8/5  (38)
(38)
An element's ability to bind to and with other elements is determined by which of the following?
(Multiple Choice)
4.9/5  (45)
(45)
How many milliequivalents are represented by a mole of bicarbonate ions (HCO₃⁻)?
(Essay)
4.8/5  (32)
(32)
Compare and contrast the chemical bonds between adjacent monomers in DNA, and between two strands of DNA.
(Essay)
4.9/5  (42)
(42)
Showing 41 - 60 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)