Exam 3: Elements, Compounds, and the Periodic Table
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
Which of these elements has the most similar chemical properties to sulfur?
(Multiple Choice)
4.9/5  (38)
(38)
How many atoms of each element appear on each side of the arrow in the following chemical equation? 3Cl3BNH2CH3 + 6(CH3)3N 6(CH3)3NHCl + B3N3Cl3(CH3)3 Hint: Distribute coefficients across the entire compound for each reactant and product. Be sure to account for parenthesis.
(Multiple Choice)
4.7/5  (38)
(38)
The alkali metals like sodium and potassium are soft metals, so they are unreactive towards water.
(True/False)
4.8/5  (38)
(38)
Write the formula of the alkane hydrocarbon with seven carbon atoms.
(Short Answer)
4.8/5  (39)
(39)
Write the formula for the compound that has the atoms and, or groups in the order given: 3 Fe, and two groups made up of 1 As and 4 O. Hint: The groups of atoms are polyatomic ions.
(Short Answer)
4.9/5  (34)
(34)
Which of these elements has the most similar chemical properties to magnesium?
(Multiple Choice)
4.9/5  (26)
(26)
Which compound exists as a diatomic molecule in its free state?
(Multiple Choice)
4.9/5  (35)
(35)
What is the formula of the compound formed from the calcium ion and HCO3-?
(Short Answer)
4.8/5  (42)
(42)
List the polyatomic ions, including the number of each type, present in the compound, (NH4)3PO4. Hint: Think about the requirements for forming ionic compounds and how this affects the number of each ion present in the formula.
(Multiple Choice)
4.7/5  (33)
(33)
Select the examples in which the formulas do not correctly match the names of the compounds indicated. 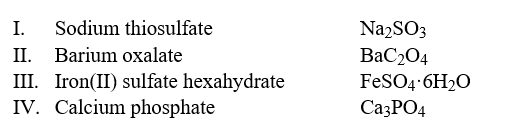 Hint: Write the correct name of each formula without looking at the names and then compare it to the choices.
Hint: Write the correct name of each formula without looking at the names and then compare it to the choices.
(Multiple Choice)
4.7/5  (39)
(39)
Which of these elements has the most similar chemical properties silicon?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 81 - 100 of 227
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)