Exam 1: A Very Brief History of Chemistry
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
A naturally occurring element consists of three isotopes. The data for the isotopes are:
Isotope 1: 147.9554 u, 10.563%
Isotope 2: 150.9496 u, 70.811%
Isotope 3: 152.9461 u, 18.626% What is the average atomic mass of this naturally occurring element?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
D
Which of the following have opposite electric charges?
Free
(Multiple Choice)
4.9/5  (24)
(24)
Correct Answer:
A
The atomic mass of naturally occurring iron, which is a mixture of isotopes, is listed as 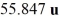 . This means that the average mass of iron is
. This means that the average mass of iron is
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
D
In stars the heaviest elements migrate to the outer layers of the star due to centrifugal forces.
(True/False)
4.9/5  (35)
(35)
Chemistry has four main ideas that were given in chapter 0. List them.
(Short Answer)
4.8/5  (40)
(40)
The relative number of atoms of each element in a particular compound
(Multiple Choice)
4.8/5  (42)
(42)
A naturally occurring element consists of two isotopes. Calculate the average atomic mass and identify the element, based on the data below.
Isotope 1: 10.013 u 19.78 %
Isotope 2: 11.009 u ------% ,Hint: Percentages should always add up to 100% then find the weighted average.
(Multiple Choice)
4.9/5  (36)
(36)
When a piece of paper burns in a closed container, the combined masses of the products is less than the mass of the original piece of paper.
(True/False)
4.9/5  (41)
(41)
A compound of hydrogen and sulfur contains 2.69 grams of hydrogen and 47.31 grams of sulfur. Another sample of the same compound that contains 75.63 grams of sulfur would contain how many grams of hydrogen? Hint: Consider the ratio of the masses to determine the mass of hydrogen
(Multiple Choice)
4.9/5  (45)
(45)
Stars that are classified as red giants are formed after the outer layer of hydrogen in a star cools and is no longer white hot.
(True/False)
4.8/5  (31)
(31)
An atom of the isotope sulfur-33 consists of how many protons, neutrons, and electrons? (p = proton, n = neutron, e = electron)
(Multiple Choice)
4.8/5  (40)
(40)
The particles found in nuclei, the protons and neutrons, are collectively called ________.
(Short Answer)
4.8/5  (35)
(35)
According to recent IUPAC recommendations, a range of atomic masses should be used instead a single value.
(True/False)
4.9/5  (36)
(36)
Molecular self-assembly occurs when two atoms can spontaneously arrange themselves into creating a diatomic molecular structure.
(True/False)
4.9/5  (35)
(35)
Consider the atoms of 59Co and 60Co. Both of these atoms have the same
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following is the logical progression of elements formed in a star?
(Multiple Choice)
4.9/5  (39)
(39)
A naturally occurring element consists of three isotopes. The data for the isotopes are:
Isotope 1: 46.972 u, 69.472%
Isotope 2: 48.961 u, 21.667%
Isotope 3: 49.954 u, 8.8610% What is the average atomic mass of this naturally occurring element?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)