Exam 6: Oxidation-Reduction Reactions
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
The oxidation number of P in HPO42- is ________.
Free
(Short Answer)
4.9/5  (35)
(35)
Correct Answer:
+5
Coal is still a large part of the world's energy supply, but not all coal is the same. Some coal deposits contain high amounts of sulfur. Explain how burning coal with high sulfur content, in form of SO2,could be harmful to the environment.
Free
(Essay)
4.8/5  (37)
(37)
Correct Answer:
The sulfur in the coal will form SO2, which can further be oxidized to SO3. In the atmosphere, SO3 reacts with water to form H2SO4 which leads to acid rain. Acid rain has many harmful effects on the environment.
What is the change in the oxidation number of carbon in the following? K2C2O4 K2CO3
Free
(Multiple Choice)
4.8/5  (45)
(45)
Correct Answer:
D
What is the change in the oxidation number of sulfur in the following?
K2SO3 K2SO4
(Multiple Choice)
4.9/5  (41)
(41)
When concentrated nitric acid behaves as an oxidizing agent, how does the oxidation number of N change? Hint: An oxidizing agent is reduced.
(Short Answer)
4.7/5  (45)
(45)
Nitric acid reacts with copper metal according to the unbalanced equation,
Cu(s)+ HNO3(aq) Cu(NO3)2(aq)+ NO2(g)+ H2O Which is correct?
(Multiple Choice)
4.9/5  (32)
(32)
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Which reaction below occurs spontaneously upon mixing the reagents shown?
(Multiple Choice)
4.8/5  (35)
(35)
After balancing the following equation for the reaction in acidic media,
H+ + HSO3-(aq)+ MnO4-(aq) MnO2(s)+ HSO4-(aq)+ H2O what is the sum of ALL the coefficients in the equation? (Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
(Multiple Choice)
4.8/5  (34)
(34)
Consider the oxidation states of hydrogen in the following compounds: LiH, NaH, KH, MgH2, CaH2, NiH, CuH, ZnH2, HF, HCl, HBr, HI, H2O, H2S, H2Se, and H2Te. What statement is true?
(Multiple Choice)
4.8/5  (35)
(35)
Ethanol, C2H5OH, is often found in gasoline. In these cases it is often produced from ethanol plants that turn corn into ethanol. The cost-benefit analysis of this process is often a topic of debate. Explain why some ethanol in gasoline could aid in the efficiency of your engine.
(Essay)
4.8/5  (34)
(34)
Consider the redox equation,
2VO43-(aq)+ SO2(g)+ 8H+(aq) 2VO2+(aq)+ SO42-(aq)+ 4H2O(l) The oxidizing agent is
(Multiple Choice)
4.9/5  (30)
(30)
Magnesium metal reacts with aqueous sulfuric acid solution to produce aqueous magnesium sulfate and hydrogen gas. In the course of the reaction, which element undergoes an increase in oxidation number?
(Multiple Choice)
4.8/5  (36)
(36)
The activity series of metals is Au < Ag < Cu < Sn < Cd < Zn < Al < Mg < Na < Cs Which reaction below does not occur spontaneously upon mixing the reagents shown?
(Multiple Choice)
4.9/5  (41)
(41)
The reaction, Cl2(g)+ NaBr(aq) NaCl(aq)+ Br2(l), involves changes in oxidation number and is therefore classified as a redox reaction.
(True/False)
4.8/5  (34)
(34)
It is proposed to measure the CO gas present in air samples by passing the gas through a solution of potassium permanganate. The reaction that occurs is, 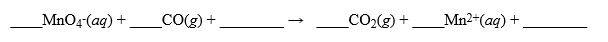 In a trial run, it required 102 liters of air containing CO (no other substances that would react with permanganate were present)to completely decolorize 250 mL of 0.0150 M MnO4-(aq)solution in a scrubber. If air weighs 1.29 g/liter, what is the percent by weight of CO in the air? Hint: Make sure your equation is balanced and pay attention to your units throughout the problem.
In a trial run, it required 102 liters of air containing CO (no other substances that would react with permanganate were present)to completely decolorize 250 mL of 0.0150 M MnO4-(aq)solution in a scrubber. If air weighs 1.29 g/liter, what is the percent by weight of CO in the air? Hint: Make sure your equation is balanced and pay attention to your units throughout the problem.
(Short Answer)
5.0/5  (33)
(33)
In a chemical reaction, one of the reactants is MnO2. It is transformed into MnSO4. What is the change in the oxidation number of the manganese?
(Multiple Choice)
4.9/5  (31)
(31)
After balancing the following equation for the reaction in acidic media,
Fe2+(aq)+ Cr2O72(aq)+ H+(aq) Cr3+(aq)+ Fe3+(aq)+ H2O(l)what is the sum of ALL the coefficients in the equation?(Remember to include values of 1 in your summation.)Hint: Start by balancing each half-reaction.
(Multiple Choice)
5.0/5  (36)
(36)
Fe2+(aq)reacts with MnO4-(aq)ion in acidic solution to yield Fe3+(aq)ions and Mn2+(aq)ions.5Fe2+(aq)+ MnO4-(aq)+ 8H+ 5 Fe3+(aq)+ Mn2+(aq)+ 4H2O Melanterite is a greenish mineral, and can be found on the walls of mines. A sample of melanterite, FeSO4·7H2O, was analyzed for purity using this reaction by titration of an aqueous solution of the sample. One such sample required 47.35 mL of 0.0175350 M permanganate solution to completely titrate all the iron in the sample by the reaction shown above. How much did the sample weigh, if it was in fact pure melanterite? Hint: Watch your units carefully at each step of the problem.
(Multiple Choice)
4.8/5  (38)
(38)
Balance the redox reaction below in basic solution.Cl2(g)→ Cl-(aq)+ ClO-(aq)What is the coefficient of OH- in the final balanced equation? Hint: Consider your oxidation numbers when balancing redox reactions.
(Short Answer)
4.8/5  (31)
(31)
Showing 1 - 20 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)