Exam 1: A Very Brief History of Chemistry
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
Which of the following statements is/are NOT consistent with Dalton's atomic theory?
I. The atoms in a given sample of an element do not share any common properties.
II. Matter consists of tiny particles called molecular substances.
III. In chemical reactions, atoms merely rearrange and do not disintegrate.
(Multiple Choice)
4.8/5  (37)
(37)
An ion of the isotope chlorine-35 has an ionic charge of 1-. How many protons, neutrons, and electrons does it contain? (p = proton, n = neutron, e = electron)Hint: Consider how changes in the number of electrons versus the number of protons affects the ionic charge of an ion.
(Multiple Choice)
4.8/5  (31)
(31)
Consider the atoms of 65Cu and 65Zn. Both of these atoms have the same
(Multiple Choice)
4.7/5  (35)
(35)
The atomic mass of naturally occurring nickel, which is a mixture of isotopes, is listed as 58.6934 u. This means that the average mass of nickel is
(Multiple Choice)
4.8/5  (35)
(35)
In a neutral atom, the number of protons must equal the number of neutrons.
(True/False)
4.9/5  (43)
(43)
In a mass spectrometer, a beam of ions emerge from between the poles of the magnet after being sorted into an array of beams based on their masses.
(True/False)
4.8/5  (37)
(37)
Which one of the following contributes to the charge of an atom, but does NOT contribute significantly to the mass of an atom?
(Multiple Choice)
4.9/5  (34)
(34)
What two factors determine the final chemical makeup of a planet?
(Short Answer)
5.0/5  (33)
(33)
When positive ions are formed in a mass spectrometer, they are attracted to a positively charged metal plate that has a small hole in its center.
(True/False)
4.7/5  (33)
(33)
A naturally occurring element consists of three isotopes. The data for the isotopes are:
Isotope 1: 146.9672 u, 64.792%
Isotope 2: 148.9638 u, 26.117%
Isotope 3: 149.9592 u, 9.0910% What is the average atomic mass of this naturally occurring element?
(Multiple Choice)
4.7/5  (29)
(29)
A compound is made of nitrogen and hydrogen in a ratio of 22.6 grams nitrogen to 4.88 grams of hydrogen. There are ________ grams of hydrogen in a sample of the compound containing 12.6 grams of nitrogen. Hint: Consider the ratio of the masses to determine the mass of hydrogen.
(Short Answer)
4.9/5  (36)
(36)
Which description below fits the 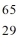
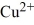 atom?Hint: Remember how charge alters the number of electrons.
atom?Hint: Remember how charge alters the number of electrons.
(Multiple Choice)
4.8/5  (37)
(37)
What is the most logical reason for only light elements being formed during the nucleosynthesis stage of the big-bang? Hint: Consider the kinetic energy of the particles and how kinetic energy can affect them coming together.
(Multiple Choice)
4.9/5  (28)
(28)
Planets are formed after supernovas from ________ left over from the formation of a new star.
(Short Answer)
4.8/5  (38)
(38)
The atomic force microscope that is used with electrically nonconducting samples makes it possible to obtain an image of individual atoms.
(True/False)
4.9/5  (45)
(45)
Which answer below best describes all atoms of a particular element?
(Multiple Choice)
4.8/5  (33)
(33)
In a mass spectrometer, a beam of ions is sorted by the magnet into a number of beams based on the same charges that they have.
(True/False)
4.9/5  (40)
(40)
Showing 21 - 40 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)