Exam 1: A Very Brief History of Chemistry
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
When an electrical spark is passed through hydrogen gas molecules, positive ions can be generated through the interaction.
(True/False)
4.9/5  (43)
(43)
Which of the following examples is consistent with the postulates from Dalton's atomic theory? Hint: Be sure to keep the definitions of atoms and molecules straight!
(Multiple Choice)
4.8/5  (37)
(37)
If 2.00 grams of molecular hydrogen react with 16.00 grams of oxygen to form water, how many grams of water must be formed if all of the hydrogen and oxygen react? Hint: Consider the ratio of the masses to determine the mass of compound.
(Short Answer)
4.8/5  (39)
(39)
A compound of phosphorus and chlorine contains 3.00 grams of phosphorus and 10.3 grams of chlorine. There are ________ grams of phosphorus in a sample of the compound containing 17.2 grams of chlorine. Hint: Consider the ratio of the masses to determine the mass of phosphorous.
(Short Answer)
4.8/5  (33)
(33)
Uranium exists in nature in the form of several isotopes and the different isotopes have different
(Multiple Choice)
5.0/5  (34)
(34)
Based on the law of Conservation of Mass, 1.8 g of elemental carbon (C)reacts with 4.8 g of molecular oxygen (O2)to produce carbon dioxide gas (CO2)as the only product. What mass of carbon dioxide is formed?
(Multiple Choice)
4.9/5  (38)
(38)
A compound is made of nitrogen and hydrogen in a ratio of 5.65 grams nitrogen to 1.22 grams of hydrogen. There are ________ grams of nitrogen in a sample of this compound containing 4.00 grams of hydrogen. Hint: Consider the ratio of the masses to determine the mass of nitrogen.
(Short Answer)
4.8/5  (36)
(36)
Atoms must be broken apart into their subatomic particles and then rearranged in order for chemical reactions to occur. Hint: What happens in a chemical reaction between reactants and products?
(True/False)
4.8/5  (34)
(34)
Compare 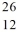 Mg and
Mg and 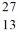 Al. In what respect do these species differ? I. number of neutrons and number of electrons
II. number of protons, and number of neutrons
III. mass number and number of protons
Al. In what respect do these species differ? I. number of neutrons and number of electrons
II. number of protons, and number of neutrons
III. mass number and number of protons
(Multiple Choice)
4.9/5  (44)
(44)
To form hydrogen sulfide, H2S, 4.03 g of molecular hydrogen (H2)are reacted with 62.13 g of sulfur (S). If all of the hydrogen and sulfur completely react to form hydrogen sulfide, how many grams of hydrogen sulfide should be formed?
(Multiple Choice)
4.9/5  (33)
(33)
Average atomic masses are used in the periodic table. These values give the true representation of mixtures of isotopes for different elements that are consistent around the world and universe.
(True/False)
4.8/5  (45)
(45)
Which of the following postulates from Dalton's atomic theory is incorrectly stated?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following particles will not be deflected by charged plates?
(Multiple Choice)
4.8/5  (36)
(36)
The atomic mass of naturally occurring gallium, which is a mixture of two isotopes, is listed as 69.723 u. This means that ,Hint: Individual atoms have a mass number that is the sum of the protons and neutrons.
(Multiple Choice)
4.8/5  (27)
(27)
Consider the atoms of 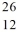 Mg and
Mg and 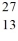 Al. Both of these species have the same
Al. Both of these species have the same
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following statements is/are consistent with Dalton's atomic theory?
I. The atoms in a given sample of an element do not share any common properties.
II. Matter consists of particles called atoms.
III. In chemical reactions, atoms merely rearrange and do not disintegrate.
(Multiple Choice)
4.7/5  (32)
(32)
Showing 61 - 80 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)