Exam 11: States of Matter
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
What is the heat of vaporization in J/g of an unknown liquid if 6823 J of heat are required to vaporize 58.0 g of the unknown at its boiling point?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following pairs of quantities is needed in calculating the amount of heat energy needed to change liquid water at 75 °C to steam at 110 °C?
(Multiple Choice)
4.9/5  (31)
(31)
How much heat energy is needed to convert 10.0 g of ice at ?10 °C to liquid water at 10 °C?
Specific heat of ice = 2.09 J/(g°C) Heat of fusion of ice = 334 J/g Specific heat of water = 4.18 J/g°C
(Multiple Choice)
4.8/5  (28)
(28)
Use a dashed line to illustrate hydrogen bonding between a methanol molecule and a hydrogen fluoride molecule.
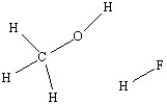
(Essay)
4.8/5  (40)
(40)
Gases are highly compressible because the particles ________.
(Multiple Choice)
4.8/5  (33)
(33)
How much energy is required to change 12.9 g of solid Cu to molten Cu at 1083 °C (melting point)?
Heat of fusion for Cu = 205 J/g
(Multiple Choice)
4.9/5  (25)
(25)
Which of the following would have the same numerical magnitude?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following types of crystalline solids would be expected to have the lowest melting point?
(Multiple Choice)
4.9/5  (39)
(39)
How much heat energy in Joules is required to heat 16.0 g of copper from 23.0 °C to 66.1 °C? Specific heat of Cu = 0.382 J/(g °C)
(Multiple Choice)
4.9/5  (34)
(34)
How much heat must be absorbed to evaporate 14 g of NH3 from liquid to gas state at ?33 °C (condensation point)? Heat of condensation for NH3 = 1380 J/g
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following quantities is needed in calculating the amount of heat energy released as water turns to ice at 0 °C?
(Multiple Choice)
4.9/5  (45)
(45)
The vapor pressure of SnCl4 reaches 400 mm Hg at 92 °C, the vapor pressure of SnI4 reaches 400 mm Hg at 315 °C, the vapor pressure of PBr3 reaches 400 mm Hg at 150 °C, and the vapor pressure of PCl3 reaches 400 mm Hg at 57 °C. At 175 °C which substance would have the lowest vapor pressure?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following would be correct units for a heat of condensation value?
(Multiple Choice)
4.8/5  (36)
(36)
In a liquid sample of NCl3, what is the dominant intermolecular force present?
(Multiple Choice)
4.9/5  (44)
(44)
A sample of aluminum absorbed 9.86 J of heat and the temperature increased from 23.2 °C to 30.5 °C. What is mass of the aluminum? The specific heat of aluminum is 0.90 J/g °C.
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following substances would be expected to have the highest boiling point?
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the mass of gold (0.130 J/g °C) that requires 468 J to heat the metal from 21.6 °C to 33.2 °C?
(Multiple Choice)
4.7/5  (34)
(34)
Showing 41 - 60 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)