Exam 11: States of Matter
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
Which of the following statements concerning the physical states of the elements at room temperature (25º C) and pressure is incorrect?
(Multiple Choice)
4.8/5  (40)
(40)
How many Joules of heat must be removed to lower the temperature of a 36.5 g Al bar from 84.1 °C to 56.8 °C? The specific heat of Al is 0.908 J/g °C.
(Multiple Choice)
4.8/5  (38)
(38)
The normal boiling point of a substance is determined by its molecular mass and its intermolecular forces. Considering these two factors, predict the order of increasing boiling points for the following substances: CH4, CS2, KBr, NH3.
(Multiple Choice)
4.8/5  (34)
(34)
What is the heat capacity of 84.0 g of water? Specific heat of water = 4.18 J/(g °C)
(Multiple Choice)
4.7/5  (34)
(34)
If liquid methyl amine and hydrofluoric acid were mixed, between which two atoms in these molecules would a hydrogen bond form?
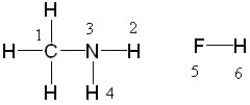
(Multiple Choice)
4.9/5  (40)
(40)
The anomalously high boiling point of water is due to ________.
(Multiple Choice)
4.7/5  (33)
(33)
A chocolate, caramel pecan piece of candy contains 115 food Calories (Cal). A food Calorie is equivalent to 1000 calories of heat energy. If the heat energy contained in the piece of candy were transferred to 53.5 kg of water at 25.0 °C, what would be the final temperature of the water? The specific heat of water is 4.18 J/g °C.
(Essay)
4.9/5  (29)
(29)
Which of the following molecules are capable of hydrogen bonding with water?
H2S NH3 H2 HBr CH
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following is not a correct characterization of the liquid state?
(Multiple Choice)
4.8/5  (42)
(42)
What would be the temperature change, in C, if 555 J of heat energy is added to 35.0 g of a metal with a specific heat of 0.418 J/g °C?
(Essay)
4.9/5  (32)
(32)
The calculation below is set up to determine how much heat is needed to convert 50 g of ice at ?20 °C to steam at 300 °C. Which constant does A represent? (A)(50 g)(20 °C) + (heat of fusion)(50 g) + (4.18 J/g°C)(50 g)(100 °C) + (B)(50 g) + (C)(50 g )(200 °C) = total heat
(Multiple Choice)
5.0/5  (41)
(41)
Which one of the following is classified as a covalent solid?
(Multiple Choice)
4.8/5  (42)
(42)
The combustion of one mole of a gas produces 212 calories of energy. Express the energy released in this reaction in kilojoules.
(Multiple Choice)
4.9/5  (33)
(33)
According to kinetic molecular theory, the molecules of a gas ________.
(Multiple Choice)
4.8/5  (42)
(42)
A T-bone steak provides 6.90 x 102 food Calories (Cal). A food Calorie is equivalent to 4180 J of heat energy. The heat energy provided by the steak would be sufficient to heat 59.1 kg of water how many Celsius degrees. The specific heat of water is 4.18 J/g °C.
(Multiple Choice)
4.9/5  (36)
(36)
Which best describes the size and shape of a sample of liquid?
(Multiple Choice)
4.7/5  (35)
(35)
On the basis of your understanding of bonding in liquids and solids, arrange the following substances from the highest to lowest melting points: NaCl Na Cl2 SiO2
(Multiple Choice)
4.8/5  (39)
(39)
The strength of London dispersion forces depends on what two factors?
(Multiple Choice)
4.8/5  (47)
(47)
The specific heat of substance A is one fourth that of substance B. The temperature of both a 24.0 g sample of substance A and a 12.0 g sample of substance B was raised 20 °C. The heat absorbed by substance A was ________ the heat absorbed by substance B.
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following statements concerning temperature change as a substance is heated is incorrect?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 21 - 40 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)