Exam 2: Numbers From Measurements
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
Express the following exponential expressions in correct scientific notation.
-430 x 10−2 ____________________
(Short Answer)
4.8/5  (47)
(47)
Carry out the following calculations. Express your answer to the proper number of significant figures.
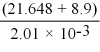
(Multiple Choice)
4.9/5  (48)
(48)
In which one of the following numbers are none of the zeros significant?
(Multiple Choice)
4.8/5  (40)
(40)
A lab technician was assigned the task of determining the density of a sample of blood plasma. The technician performed three replicate measurements of the density of the sample and reported the following results:
0.810 g/mL, 1.375 g/mL, 2.046 g/mL
The actual density of blood plasma is 1.027 g/mL. In evaluating the technician's job performance in terms of accuracy and precision, it can be said that the technician ________.
(Multiple Choice)
4.9/5  (36)
(36)
Do the following multiplications and divisions, expressing your answers to the proper number of significant figures.
-(7.0 x 10-6) x (3.00 x 104) ____________________
(Short Answer)
4.9/5  (37)
(37)
Which one of the following numbers contains 4 significant figures?
(Multiple Choice)
4.9/5  (37)
(37)
Using scientific notation, express the number five million five hundred thousand to 4 significant figures.
(Short Answer)
4.9/5  (39)
(39)
Round off each of the following numbers to 3 significant figures.
-1900 ____________________
(Short Answer)
4.7/5  (35)
(35)
Express the following numbers in scientific notation.
-6,473 ____________________
(Short Answer)
4.8/5  (35)
(35)
Which measuring device below is the most accurate for measuring volume?
(Multiple Choice)
4.9/5  (46)
(46)
Perform the following mathematical operations and express the answers to the correct number of significant figures.
-[10-7 x 106 x 104] / [10-5 x 109] = ____________________
(Short Answer)
4.8/5  (33)
(33)
Which of the following contain three significant figures?
I. 326.0
II. 0.00310
III. 46,900
IV. 1.070
V. 0.020
(Multiple Choice)
4.9/5  (36)
(36)
If you were recording the volume of liquid in the graduated cylinder depicted, what volume would you record (to the correct number of significant figures)?
(Multiple Choice)
4.8/5  (43)
(43)
Round off the following numbers to 4 significant figures.
-15000 ____________________
(Short Answer)
4.7/5  (32)
(32)
The correct answer obtained by dividing 4.65 x 105 by 9.4 x 10?2 together is ________.
(Multiple Choice)
4.8/5  (23)
(23)
If the accepted value for the length of an object is 6.78 cm, which of the following sets of experimental results is best described as both precise and accurate?
(Multiple Choice)
4.8/5  (38)
(38)
Round off the following numbers to 4 significant figures.
-398.845 ____________________
(Short Answer)
4.8/5  (36)
(36)
Do the following additions or subtractions, expressing your answers to the proper number of significant figures.
-3.070 − 3.050 ____________________
(Short Answer)
5.0/5  (37)
(37)
Round off each of the following numbers to the number of significant figures indicated in parenthesis.
-0.45 (one) ____________________
(Short Answer)
4.8/5  (42)
(42)
The number 3009.1 expressed in scientific notation to the correct number of significant figures becomes ________.
(Multiple Choice)
4.9/5  (38)
(38)
Showing 21 - 40 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)