Exam 2: Numbers From Measurements
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
How many numbers will be in the coefficient when 0.0090110 is expressed in scientific notation?
(Multiple Choice)
4.7/5  (44)
(44)
Do the following additions or subtractions, expressing your answers to the proper number of significant figures.
-(6.3 x 107) + (4.5 x 103) ____________________
(Short Answer)
4.9/5  (39)
(39)
Perform the following mathematical operations and express the answers to the correct number of significant figures.
-(235.8 + 15940 + 6.17) ÷ 1.987 = ____________________
(Short Answer)
4.8/5  (45)
(45)
A balance has an accuracy of ±0.01 grams. A beaker weighed 15 grams when weighed on this balance. Using the correct number of significant figures, the weight of the beaker should be recorded as ________.
(Multiple Choice)
4.9/5  (40)
(40)
Round off each of the following numbers to 3 significant figures.
-0.00860 ____________________
(Short Answer)
4.8/5  (35)
(35)
Round off each of the following numbers to the number of significant figures indicated in parenthesis.
-431.50 (three) ____________________
(Short Answer)
4.9/5  (35)
(35)
Express the following numbers in scientific notation.
-623,000,000 ____________________
(Short Answer)
4.9/5  (33)
(33)
What is the correct exponential term for the following mathematical operation?
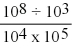
(Multiple Choice)
4.7/5  (37)
(37)
For each of the calculator-completed calculations on the left, determine the correct number of significant figures that the answer should have. Give your response using the choices on the right.

(Essay)
4.7/5  (36)
(36)
Do the following calculation and express the answer using the correct scientific notation.
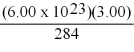
(Multiple Choice)
4.7/5  (35)
(35)
Convert the following numbers from scientific notation to ordinary decimal notation.
-9.0 x 10−3 ____________________
(Short Answer)
4.9/5  (36)
(36)
Do the following calculation. How many significant figures are justified for the answer?
5.02 + 6.119 + 0.04117
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following sources of experimental error can be classified as random error?
(Multiple Choice)
4.9/5  (38)
(38)
Round off the following numbers to 4 significant figures.
-245,864 ____________________
(Short Answer)
4.9/5  (32)
(32)
Do the following additions or subtractions, expressing your answers to the proper number of significant figures.
-4.63 + 7.014 − 1.200 ____________________
(Short Answer)
4.8/5  (40)
(40)
Water and vitamin C were added to a beaker. Calculate the mass of the beaker and its contents, and choose the answer with the appropriate number of significant figures.
146.20 g beaker + 23.1 g water + 0.34 g vitamin C =
(Multiple Choice)
4.8/5  (40)
(40)
Do the following multiplications and divisions, expressing your answers to the proper number of significant figures.
-86.40/12.095 ____________________
(Short Answer)
4.9/5  (39)
(39)
If you were recording the volume of liquid in the pipet depicted, what volume would you record (to the correct number of significant figures)?
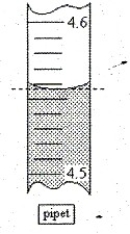
(Multiple Choice)
4.8/5  (39)
(39)
Which one of the following mathematical expressions is not evaluated correctly?
(Multiple Choice)
4.9/5  (33)
(33)
The number 1.987 x 106 in normal decimal notation is ________.
(Multiple Choice)
4.7/5  (36)
(36)
Showing 41 - 60 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)