Exam 2: Numbers From Measurements
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
Perform the following mathematical operations. Express your answer to the proper number of significant figures.
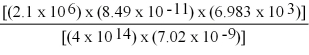
(Short Answer)
5.0/5  (46)
(46)
Convert the following numbers from scientific notation to ordinary decimal notation.
-6.429 x 108 ____________________
(Short Answer)
4.8/5  (44)
(44)
Express the following numbers in scientific notation.
-0.00021 ____________________
(Short Answer)
4.9/5  (38)
(38)
The number 12.68 x 102, when expressed in correct scientific notation, becomes ________.
(Multiple Choice)
4.8/5  (38)
(38)
Which measurement is consistent with a graduated cylinder which has an uncertainty of 0.1 mL?
(Multiple Choice)
4.9/5  (40)
(40)
How many significant figures are found in each of the following measurements?

(Essay)
4.7/5  (42)
(42)
A rubber band is found to weigh 0.0978 g. What is the total mass of 106 such identical rubber bands?
(Multiple Choice)
4.9/5  (45)
(45)
How many of the following numbers have 4 significant figures?
19.00 0.00006 1.609 x 108 13,600
(Multiple Choice)
4.8/5  (44)
(44)
The number 0.00309 expressed in scientific notation to the correct number of significant figures becomes ________.
(Multiple Choice)
4.8/5  (42)
(42)
A student cut 1200 pieces of copper wire, each wire weighed 1.769 grams. Calculate the total mass of the pieces of copper to the correct number of significant figures.
(Multiple Choice)
4.9/5  (41)
(41)
The number 0.090804, when rounded off to 4 significant figures, would appear as ________.
(Multiple Choice)
4.7/5  (38)
(38)
Express the following exponential expressions in correct scientific notation.
-0.123 x 106 ____________________
(Short Answer)
4.7/5  (44)
(44)
Do the following multiplications and divisions, expressing your answers to the proper number of significant figures.
-(8.00 x 106)/(4.00 x 104) ____________________
(Short Answer)
4.8/5  (34)
(34)
Do the following multiplications and divisions, expressing your answers to the proper number of significant figures.
-(2.00 x 102) x (2.00 x 10-4) ____________________
(Short Answer)
4.7/5  (34)
(34)
Perform the following mathematical operations and express the answers to the correct number of significant figures.
-[(3.9780 x 10-2) x (1.010 x 104)]/ [(3.290 x 10-5) x (7.85 x 102)] = ___________________
(Short Answer)
4.8/5  (43)
(43)
Express the following numbers in scientific notation.
-0.005300 ____________________
(Short Answer)
4.9/5  (45)
(45)
When 0.0005760 is written in proper scientific notation with the correct number of significant figures the number is:
(Multiple Choice)
4.9/5  (38)
(38)
Express the following exponential expressions in correct scientific notation.
-0.000330 x 10−2 ____________________
(Short Answer)
4.9/5  (40)
(40)
Showing 81 - 99 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)