Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism
Exam 1: Biochemistry and the Organization of Cells69 Questions
Exam 2: Water: The Solvent for Biochemical Reactions79 Questions
Exam 3: Amino Acids and Peptides77 Questions
Exam 4: The Three-Dimensional Structure of Proteins77 Questions
Exam 5: Protein Purification and Characterization Techniques58 Questions
Exam 6: The Behavior of Proteins: Enzymes78 Questions
Exam 7: The Behavior of Proteins: Enzymes, Mechanisms, and Control77 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes90 Questions
Exam 9: Nucleic Acids: How Structure Conveys Information60 Questions
Exam 10: Biosynthesis of Nucleic Acids: Replication79 Questions
Exam 11: Transcription of the Genetic Code: Biosynthesis of Rna93 Questions
Exam 12: Protein Synthesis: Translation of the Genetic Message80 Questions
Exam 13: Nucleic Acid Biotechnology Techniques89 Questions
Exam 14: Viruses, Cancer, and Immunology35 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism56 Questions
Exam 16: Carbohydrates88 Questions
Exam 17: Glycolysis63 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism79 Questions
Exam 19: The Citric Acid Cycle77 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation70 Questions
Exam 21: Lipid Metabolism86 Questions
Exam 22: Photosynthesis74 Questions
Exam 23: The Metabolism of Nitrogen78 Questions
Exam 24: Integration of Metabolism: Cellular Signaling60 Questions
Select questions type
In order to drive the synthesis of ATP,the hydrolysis of an organic phosphate
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
A
I am performing a reaction,A B,with DG°' = -0.3 kJ/mol.I start the reaction with 10 mM A and no B.After allowing the reaction to proceed for 24 hrs at room temperature and atmospheric pressure,I analyze a sample of the reaction mix to find I now have 1 mM A and 9 mM B.Which of the following conclusions should I make?
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
D
The number of degrees of freedom a molecule has is related to the numbers of resonant structures it has.
(True/False)
4.8/5  (29)
(29)
Consider these reactions:  What is the DG° of the last reaction?
What is the DG° of the last reaction?
(Multiple Choice)
4.8/5  (31)
(31)
The production of larger molecules from smaller ones is called
(Multiple Choice)
4.9/5  (36)
(36)
If the reaction A B has DG = +25 Joule/mol and the reaction B C has DG = -15 Joule/mol,the overall energy change A C will be
(Multiple Choice)
4.8/5  (37)
(37)
Exhibit 15A 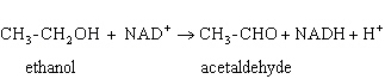 Consider the reaction of alcohol dehydrogenase.
Refer to Exhibit 15A.Which molecule loses electrons?
Consider the reaction of alcohol dehydrogenase.
Refer to Exhibit 15A.Which molecule loses electrons?
(Multiple Choice)
5.0/5  (40)
(40)
When we say that the efficiency of glycolysis is about 33% we mean that
(Multiple Choice)
4.9/5  (34)
(34)
The conversion of NAD+ to NADH is an example of reduction because
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following is not a mechanism used to activate substrates for further metabolism?
(Multiple Choice)
4.9/5  (35)
(35)
In the coenzyme FAD the site to which electrons are transferred is
(Multiple Choice)
4.7/5  (36)
(36)
Biochemists use a modified value for standard DG values because
(Multiple Choice)
4.9/5  (33)
(33)
The body allows energy consuming reactions to occur by coupling them with reactions which have a negative DG.
(True/False)
4.8/5  (36)
(36)
In general,the anabolic pathways tend to involve oxidation reactions.
(True/False)
4.9/5  (40)
(40)
Which best describes the DG for hydrolysis of creatine phosphate under cellular conditions in which the concentration of creatine phosphate,creatine,and phosphate all equal 1 mM at 25°C.The DG° for the hydrolysis of creatine phosphate at 25°C is -43 kJ/mol.
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)