Exam 18: Aqueous Ionic Equilibrium
Exam 1: Essentials: Units, Measurements, and Problem Solving101 Questions
Exam 2: Atoms137 Questions
Exam 3: The Quantum Mechanical Model of the Atom141 Questions
Exam 4: Periodic Properties of the Elements144 Questions
Exam 5: Molecules and Compounds202 Questions
Exam 6: Chemical Bonding I138 Questions
Exam 7: Chemical Bonding Ii64 Questions
Exam 8: Chemical Reactions and Chemical Quantities79 Questions
Exam 9: Introduction to Solutions and Aqueous Reactions177 Questions
Exam 10: Thermochemistry145 Questions
Exam 11: Gases185 Questions
Exam 12: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 13: Crystalline Solids and Modern Materials43 Questions
Exam 14: Solutions148 Questions
Exam 15: Chemical Kinetics155 Questions
Exam 16: Chemical Equilibrium141 Questions
Exam 17: Acids and Bases159 Questions
Exam 18: Aqueous Ionic Equilibrium188 Questions
Exam 19: Free Energy and Thermodynamics130 Questions
Exam 20: Electrochemistry148 Questions
Exam 21: Radioactivity and Nuclear Chemistry136 Questions
Exam 22: Organic Chemistry104 Questions
Exam 23: Transition Metals and Coordination Compounds74 Questions
Select questions type
The molar solubility of Ba3(PO4)2 is 8.89 × 10-9 M in pure water.Calculate the Ksp for Ba3(PO4)2.
(Multiple Choice)
4.8/5  (33)
(33)
What is the molar solubility of AgCl in 0.30 M NH3? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(NH3)2+ is 1.7 × 107.
(Multiple Choice)
4.8/5  (35)
(35)
A 25.0 mL sample of 0.150 M hydrofluoric acid is titrated with a 0.150 M NaOH solution.What is the pH after 26.0 mL of base is added? The Ka of hydrofluoric acid is 6.8 × 10-4.
(Multiple Choice)
4.9/5  (42)
(42)
Calculate the pH of a solution that is 0.111 M in sodium formate (NaHCO2)and 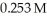 in formic acid (HCO2H).The Ka of formic acid is 1.77 × 10-4.
in formic acid (HCO2H).The Ka of formic acid is 1.77 × 10-4.
(Multiple Choice)
4.7/5  (30)
(30)
You wish to prepare an HC2H3O2 buffer with a pH of 5.24.If the pKa of is 4.74,what ratio of C2H3O2⁻/HC2H3O2 must you use?
(Multiple Choice)
4.8/5  (52)
(52)
A sample contains Ba3(PO4)2, CdS,AgCl,NH4Cl,and ZnS.Identify the precipitate after the addition of 6 M HCl.
(Multiple Choice)
4.8/5  (42)
(42)
Formic acid (HCO2H,Ka = 1.8 × 10-4)is the principal component in the venom of stinging ants.What is the molarity of a formic acid solution if 25.00 mL of the formic acid solution requires 29.80 mL of 0.0567 M NaOH to reach the equivalence point?
(Multiple Choice)
4.9/5  (38)
(38)
What is the molar solubility of Mg(OH)2 in a basic solution with a pH of 12.50? Ksp for Mg(OH)2 is 5.6 × 10-12.
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the pH of a solution formed by mixing 250.0 mL of 0.15 M HCHO2 with 100.0 mL of 0.20 M LiCHO2.The Ka for HCHO2 is 1.8 × 10-4.
(Multiple Choice)
4.8/5  (37)
(37)
A 25.0-mL sample of 0.150 M benzoic acid is titrated with a 0.150 M NaOH solution.What is the pH before any base is added? The Ka of benzoic acid is 6.3 × 10-5.
(Multiple Choice)
4.9/5  (42)
(42)
A 25.0-mL sample of 0.150 M hydrocyanic acid is titrated with a 0.150 M NaOH solution.What is the pH after 13.3 mL of base is added? The Ka of hydrocyanic acid is 4.9 × 10-10.
(Multiple Choice)
4.8/5  (29)
(29)
Determine the molar solubility for Al(OH)3 in pure water.Ksp for Al(OH)3 = 1.3 × 10-33.
(Multiple Choice)
4.9/5  (42)
(42)
Give the expression for the solubility product constant for Cr2(CO3)3.
(Multiple Choice)
4.7/5  (30)
(30)
Determine the molar solubility for Cd(OH)2 in pure water.Ksp for Cd(OH)2 is 2.0 × 10-14.
(Multiple Choice)
4.9/5  (29)
(29)
Sodium hypochlorite,NaOCl,is the active ingredient in household bleach.What is the concentration of hypochlorite ion if 20.00 mL of bleach requires 32.00 mL of 0.500 M HCl to reach the equivalence point?
(Multiple Choice)
4.9/5  (34)
(34)
Give the equation for a saturated solution in comparing Q with Ksp.
(Multiple Choice)
4.7/5  (42)
(42)
A 100.0 mL sample of 0.20 M HF is titrated with 0.10 M KOH.Determine the pH of the solution after the addition of 300.0 mL of KOH.The Ka of HF is 3.5 × 10-4.
(Multiple Choice)
5.0/5  (40)
(40)
Calculate the pH of a solution formed by mixing 200.0 mL of 0.30 M HClO with 100.0 mL of 0.20 M KClO.The Ka for HClO is 2.9 × 10-8.
(Multiple Choice)
4.8/5  (36)
(36)
Showing 121 - 140 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)