Exam 24: Introduction to Analytical Separations
Exam 1: The Analytical Process20 Questions
Exam 2: Chemical Measurements21 Questions
Exam 3: Tools of the Trade19 Questions
Exam 4: Experimental Error20 Questions
Exam 5: Statistics22 Questions
Exam 6: Quality Assurance and Calibration Methods20 Questions
Exam 7: Chemical Equilibrium20 Questions
Exam 8: Let the Titrations Begin20 Questions
Exam 9: Activity and the Systematic Treatment of Equilibrium20 Questions
Exam 10: Monoprotic Acid-Base Equilibria20 Questions
Exam 11: Polyprotic Acid-Base Equilibria20 Questions
Exam 12: Acid-Base Titrations20 Questions
Exam 13: Edta Titrations20 Questions
Exam 14: Advanced Topics in Equilibrium20 Questions
Exam 15: Fundamentals of Electrochemistry20 Questions
Exam 16: Electrodes and Potentiometry20 Questions
Exam 17: Redox Titrations20 Questions
Exam 18: Electroanalytical Techniques20 Questions
Exam 19: Fundamentals of Spectrophotometry23 Questions
Exam 20: Applications of Spectrophotometry20 Questions
Exam 21: Spectrophotometers20 Questions
Exam 22: Atomic Spectroscopy20 Questions
Exam 23: Mass Spectrometry20 Questions
Exam 24: Introduction to Analytical Separations20 Questions
Exam 25: Gas Chromatography20 Questions
Exam 26: High-Performance Liquid Chromatography20 Questions
Exam 27: Chromatographic Methods and Capillary Electrophoresis20 Questions
Exam 28: Gravimetric and Combustion Analysis20 Questions
Exam 29: Sample Preparation19 Questions
Select questions type
____________________ is a plot of detector response versus retention time.
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
C
Which comparison of open tubular columns to packed columns is INCORRECT?
Free
(Multiple Choice)
4.8/5  (25)
(25)
Correct Answer:
E
Calculate the weak acid,HA,concentration that remains in the aqueous phase when 50.0 mL of 0.184 M HA at pH = 4.75 is extracted with 50 mL of toluene three times.The partition coefficient for the extraction is 5.17,favoring toluene.The Ka for HA is 8.0 x 10−4.
Free
(Essay)
4.8/5  (39)
(39)
Correct Answer:
72.5% remains or 0.133 M HA remains in the aqueous phase.
____________________ is the physical transfer of solute from one phase to another.
(Multiple Choice)
4.8/5  (36)
(36)
For two separated solutes the = 1.10 and the k2 = 4.38.How many theoretical plates are required to achieve a resolution of 1.5?
(Short Answer)
4.8/5  (41)
(41)
The van Deemter equation describes plate height in terms of constants A,B,C,and the linear velocity,ux.Which statement is NOT true for the van Deemter equation? 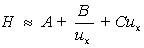
(Multiple Choice)
4.9/5  (34)
(34)
Which statements are NOT true regarding chromatography?
I The stationary phase is typically a liquid bonded to the inside surface of a capillary or the surface of solid packed in the column.
II The mobile phase entering the column is the eluate.
III Mobile phase is the solvent (gas or liquid)that moves through the column.
IV Eluent is the process of passing mobile phase though a column.
V Columns are either open tubular or packed.
(Multiple Choice)
4.7/5  (37)
(37)
The resolution between two peaks is defined as resolution = 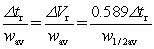 .Which variable is INCORRECTLY defined?
.Which variable is INCORRECTLY defined?
(Multiple Choice)
4.9/5  (32)
(32)
The observed variance, 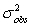 ,for a peak is the sum of the variances from all contributing factors.Which of the following is not a contributing factor to
,for a peak is the sum of the variances from all contributing factors.Which of the following is not a contributing factor to 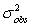 ?
?
(Multiple Choice)
4.8/5  (33)
(33)
The partition coefficient is modified when the pH of the aqueous phase is adjusted or when a chelating agent is added.The partition coefficient is renamed the distribution coefficient.What is the impact on the extraction of a weak acid into an organic solvent as the pH of the aqueous phase increases?
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the resolution for two chromatographic peaks that elute at tr1 = 1770 s and tr2 = 1800 s with base peak widths of w1 = 51 s and w2 = 57 s.
(Short Answer)
4.7/5  (40)
(40)
Which is true for plate height?
I The smaller the plate height,the narrower the peaks.
II The larger the plate height,the better separation between peaks.
III Plate height can be calculated from the column length,L,and the number of theoretical plates,N.
IV For fixed number of theoretical plates,N,the longer a solute is on column,the wider the peak will become.
(Multiple Choice)
5.0/5  (38)
(38)
A solute is to be extracted using 150 mL of solvent.Of the options below,which will extract the maximum amount of solute?
(Multiple Choice)
4.8/5  (31)
(31)
Calculate the number of theoretical plates and plate height for a peak with a retention time of 1295 seconds,a width at the baseline of 27 seconds and elutes from a 15 m column.
(Short Answer)
4.7/5  (36)
(36)
The relative retention between two solutes,1 and 2,is = 1.35.If the adjusted retention time for solute 2 is 293 s,what is adjusted retention time for solute 1?
(Multiple Choice)
4.9/5  (38)
(38)
Suppose that the partition coefficient for an amine,B,is K = 3.0 and the acid dissociation constant for BH+ is Ka = 1.0 x 10−9.If 50.0 mL of 0.010 M aqueous amine is extracted with 100.0 mL of solvent two times,what percentage amine remains in aqueous solution if the pH of the aqueous phase is 9.00?
(Short Answer)
4.8/5  (44)
(44)
Which is NOT true when scaling up preparative chromatography?
(Multiple Choice)
4.7/5  (27)
(27)
The longer an analyte remains on the column,the broader the peak becomes.Peaks widen with time on column due to :
(Multiple Choice)
4.9/5  (38)
(38)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)