Exam 29: Sample Preparation
Exam 1: The Analytical Process20 Questions
Exam 2: Chemical Measurements21 Questions
Exam 3: Tools of the Trade19 Questions
Exam 4: Experimental Error20 Questions
Exam 5: Statistics22 Questions
Exam 6: Quality Assurance and Calibration Methods20 Questions
Exam 7: Chemical Equilibrium20 Questions
Exam 8: Let the Titrations Begin20 Questions
Exam 9: Activity and the Systematic Treatment of Equilibrium20 Questions
Exam 10: Monoprotic Acid-Base Equilibria20 Questions
Exam 11: Polyprotic Acid-Base Equilibria20 Questions
Exam 12: Acid-Base Titrations20 Questions
Exam 13: Edta Titrations20 Questions
Exam 14: Advanced Topics in Equilibrium20 Questions
Exam 15: Fundamentals of Electrochemistry20 Questions
Exam 16: Electrodes and Potentiometry20 Questions
Exam 17: Redox Titrations20 Questions
Exam 18: Electroanalytical Techniques20 Questions
Exam 19: Fundamentals of Spectrophotometry23 Questions
Exam 20: Applications of Spectrophotometry20 Questions
Exam 21: Spectrophotometers20 Questions
Exam 22: Atomic Spectroscopy20 Questions
Exam 23: Mass Spectrometry20 Questions
Exam 24: Introduction to Analytical Separations20 Questions
Exam 25: Gas Chromatography20 Questions
Exam 26: High-Performance Liquid Chromatography20 Questions
Exam 27: Chromatographic Methods and Capillary Electrophoresis20 Questions
Exam 28: Gravimetric and Combustion Analysis20 Questions
Exam 29: Sample Preparation19 Questions
Select questions type
____________________ dissolves substances that will not dissolve in acid in a hot,molten inorganic____________________.
Free
(Multiple Choice)
4.9/5  (25)
(25)
Correct Answer:
B
All of the following are used to grind solids into a fine powder,except:
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
E
How many replicates must be completed for sampling deviation of 3.5% to achieve a 1% standard deviation?
Free
(Short Answer)
4.8/5  (32)
(32)
Correct Answer:
50
Which of the following is INCORRECT for decomposition of organic substances?
(Multiple Choice)
4.9/5  (34)
(34)
____________________ is the procedure in which analyte is chemically modified to make the compound easier to detect or separate.
(Multiple Choice)
4.9/5  (34)
(34)
A mixture contains 5% NaCl particles and 95% NaBr particles.If 103 particles are taken,what is the relative standard deviation for NaCl and NaBr?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following are NOT liquid extraction techniques?
I emulsive liquid-liquid microextraction
II dispersive liquid-liquid microextraction
III solid-supported liquid-liquid extraction
IV supercritical fluid extraction
V solid-liquid microextraction
(Multiple Choice)
4.7/5  (39)
(39)
All of the following are true for dissolving inorganic materials in acid,except:
(Multiple Choice)
4.8/5  (42)
(42)
Sample storage is an important aspect of sample preparation.Which is NOT true for sample storage?
(Multiple Choice)
4.8/5  (31)
(31)
What mass of a solid sample containing 2.5% analyte particles and 97.5% solvent particles must be taken to give a standard deviation of 2% of the number of analyte particles,if the average particle volume is 2.54 nL and the sample has an average density of 1.548 g/mL?
(Short Answer)
4.8/5  (32)
(32)
What mass will give a sampling standard deviation of 3.5% for a sampling constant of 40.9?
(Short Answer)
4.8/5  (36)
(36)
A mixture contains 2.5% KBr and 97.5% inert particles.What is the relative standard deviation for KBr is 50 000 particles are taken?
(Short Answer)
4.8/5  (28)
(28)
Calculate the overall standard deviation if the analytical standard deviation is 6% and the sampling standard deviation is 4%.
(Short Answer)
4.9/5  (31)
(31)
To calculate the number of required replicates for analysis the equation 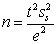 is used.Which statement is NOT true for the equation and calculation?
is used.Which statement is NOT true for the equation and calculation?
(Multiple Choice)
4.9/5  (34)
(34)
Sample preparation of a laboratory sample can involve all of the following,except:
(Multiple Choice)
4.9/5  (38)
(38)
A 4.17 g sample has a 4% sampling standard deviation.What mass sample must be taken to decrease the sampling standard deviation to 2%?
(Multiple Choice)
4.9/5  (33)
(33)
________________________________________uses a small volume of chromatographic stationary phase or molecularly imprinted polymer to isolate analytes from the sample matrix to simply analysis.
(Multiple Choice)
4.8/5  (34)
(34)
For random errors,the overall variance, 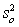 ,is calculated from the analytical variance,
,is calculated from the analytical variance, 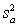 ,and the sampling variance,
,and the sampling variance, 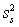 .Which is the correct equation for calculating the overall variance?
.Which is the correct equation for calculating the overall variance?
(Multiple Choice)
4.7/5  (34)
(34)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)