Exam 4: Experimental Error
Exam 1: The Analytical Process20 Questions
Exam 2: Chemical Measurements21 Questions
Exam 3: Tools of the Trade19 Questions
Exam 4: Experimental Error20 Questions
Exam 5: Statistics22 Questions
Exam 6: Quality Assurance and Calibration Methods20 Questions
Exam 7: Chemical Equilibrium20 Questions
Exam 8: Let the Titrations Begin20 Questions
Exam 9: Activity and the Systematic Treatment of Equilibrium20 Questions
Exam 10: Monoprotic Acid-Base Equilibria20 Questions
Exam 11: Polyprotic Acid-Base Equilibria20 Questions
Exam 12: Acid-Base Titrations20 Questions
Exam 13: Edta Titrations20 Questions
Exam 14: Advanced Topics in Equilibrium20 Questions
Exam 15: Fundamentals of Electrochemistry20 Questions
Exam 16: Electrodes and Potentiometry20 Questions
Exam 17: Redox Titrations20 Questions
Exam 18: Electroanalytical Techniques20 Questions
Exam 19: Fundamentals of Spectrophotometry23 Questions
Exam 20: Applications of Spectrophotometry20 Questions
Exam 21: Spectrophotometers20 Questions
Exam 22: Atomic Spectroscopy20 Questions
Exam 23: Mass Spectrometry20 Questions
Exam 24: Introduction to Analytical Separations20 Questions
Exam 25: Gas Chromatography20 Questions
Exam 26: High-Performance Liquid Chromatography20 Questions
Exam 27: Chromatographic Methods and Capillary Electrophoresis20 Questions
Exam 28: Gravimetric and Combustion Analysis20 Questions
Exam 29: Sample Preparation19 Questions
Select questions type
____________________ expresses the margin of uncertainty associated with a measurement.
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
E
The distance between two cities is measured and the reported value with ambiguous significant digits is:
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
D
The density of a solution is determined using a class A 25-mL volumetric flask and an analytical balance.If the mass of the empty volumetric flask is 26.9872 ± 0.0003 g,the mass of the flask filled with solution is 53.9820 ± 0.0003 g,and the tolerance for the flask is ± 0.03 mL,what is the density of the solution?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
D
For which measurement has the number of significant digits been incorrectly determined?
(Multiple Choice)
4.9/5  (33)
(33)
Calculate the molar mass of diethyl ether,CH3CH2OCH2CH3.(C = 12.0106 ± 0.0010,H = 1.00798 ± 0.00014,and O = 15.9990 ± 0.0008).
(Short Answer)
4.8/5  (40)
(40)
____________________ cannot be eliminated but it may be reduced by a better experiment.
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the volume of an aquarium that is 73.1 cm long,30.1 cm deep,and 25.44 cm tall.
(Multiple Choice)
4.8/5  (37)
(37)
For the paired statements below,which are TRUE for the detection of an analytical methods systematic error? 
(Multiple Choice)
4.9/5  (43)
(43)
For a fixed absolute uncertainty,as the magnitude of the measurement____________________ ,the percent relative uncertainty____________________ .
(Multiple Choice)
4.8/5  (40)
(40)
An irregular solid with a mass of 25.9863 ± 0.0003 g displaces 20.5 ± 0.2 mL of water.Calculate the density of the solid.
(Short Answer)
4.9/5  (35)
(35)
Calculate the error for the molar mass of acetic acid,CH3CO2H.The atomic masses for each element are C = 12.0106 ± 0.0010,H = 1.00798 ± 0.00014,and O = 15.9990 ± 0.0008.
(Multiple Choice)
4.8/5  (42)
(42)
Calculate the mass of a heterogeneous mixture containing 139.32 grams sand,34.99 grams gravel,and 9.372 grams salt.
(Multiple Choice)
4.7/5  (46)
(46)
For significant digits and calculations,the following statements are true,EXCEPT for which one(s)?
I Addition and subtraction―round off the answer according to the number of decimal places in the number with the most decimal places.
II Multiplication and division―the answer is limited to number of digits in the number with the fewest significant digits.
III Logarithms and antilogarithms ―the number of significant figures in the antilogarithm should equal the number of digits in the mantissa.
(Multiple Choice)
5.0/5  (37)
(37)
____________________ is a consistent error that can be detected and corrected.
(Multiple Choice)
4.8/5  (33)
(33)
Two students are tasked with determining the milligrams of chloride in a simulated soil sample.Both students extract chloride into aqueous solution and perform triplicate titration with silver nitrate solution and dichlorofluorescein indicator.The students report the following results to their laboratory instructor. 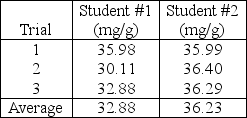 If the accepted value is 36.90 mg chloride/g sample,the laboratory instructor infers that student #2's work exhibits____________________ student #1.
If the accepted value is 36.90 mg chloride/g sample,the laboratory instructor infers that student #2's work exhibits____________________ student #1.
(Multiple Choice)
4.7/5  (43)
(43)
When calculating the uncertainty associated with molar mass,which of the following statements are TRUE? I Uncertainty in the mass of n identical atoms = n (uncertainty in atomic mass for one atom. )
II Uncertainties for different elements are dependent.
III The uncertainty for the sum of the masses of different elements is calculated using the equation 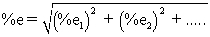 .
IV When summing the masses of different elements,the uncertainties for the mass of different elements are propagated as random errors.
.
IV When summing the masses of different elements,the uncertainties for the mass of different elements are propagated as random errors.
(Multiple Choice)
4.9/5  (41)
(41)
Calculate the mass of a concrete slab that is 51.0 cm long,17.34 cm deep,and 6.2 cm tall with a density of 5.3 g/cm3.
(Multiple Choice)
5.0/5  (37)
(37)
A mass of approximately 10 grams is measured using weight by difference.If a vial containing calcium carbonate powder has an initial mass of 87.36 ± 0.03 g and a final mass of 76.99 ± 0.03 g,what mass of calcium carbonate was taken?
(Multiple Choice)
4.8/5  (31)
(31)
Two students are tasked with determining the milligrams of chloride in a simulated soil sample.Both students extract chloride into aqueous solution and perform triplicate titration with silver nitrate solution and dichlorofluorescein indicator.The students report the following results to their laboratory instructor. 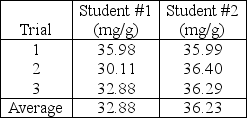 If the accepted value is 36.90 mg chloride/g sample,the laboratory instructor infers that student #1's work exhibits____________________ student #2.
If the accepted value is 36.90 mg chloride/g sample,the laboratory instructor infers that student #1's work exhibits____________________ student #2.
(Multiple Choice)
4.9/5  (38)
(38)
To the correct number of significant digits,what is the molarity of a sodium hydroxide solution when 12.9 grams of NaOH is dissolved in enough water to prepare 0.500 L of solution? (NaOH = 40.00 g/mol)
(Short Answer)
4.9/5  (33)
(33)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)