Exam 16: Temperature and Heat
Exam 1: Introduction to Physics100 Questions
Exam 2: One-Dimensional Kinematics112 Questions
Exam 3: Vectors in Physics82 Questions
Exam 4: Two-Dimensional Kinematics95 Questions
Exam 5: Newtons Laws of Motion101 Questions
Exam 6: Applications of Newtons Laws105 Questions
Exam 7: Work and Kinetic Energy92 Questions
Exam 8: Potential Energy and Conservation of Energy99 Questions
Exam 9: Linear Momentum and Collisions102 Questions
Exam 10: Rotational Kinematics and Energy102 Questions
Exam 11: Rotational Dynamics and Static Equilibrium97 Questions
Exam 12: Gravity94 Questions
Exam 13: Oscillations About Equilibrium102 Questions
Exam 14: Waves and Sound104 Questions
Exam 15: Fluids107 Questions
Exam 16: Temperature and Heat103 Questions
Exam 17: Phases and Phase Changes100 Questions
Exam 18: The Laws of Thermodynamics97 Questions
Exam 19: Electric Charges, Forces, and Fields88 Questions
Exam 20: Electric Potential and Electric Potential Energy99 Questions
Exam 21: Electric Current and Direct-Current Circuits99 Questions
Exam 22: Magnetism101 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction99 Questions
Exam 24: Alternating-Current Circuits93 Questions
Exam 25: Electromagnetic Waves90 Questions
Exam 26: Geometrical Optics92 Questions
Exam 27: Optical Instruments102 Questions
Exam 28: Physical Optics: Interference and Diffraction93 Questions
Exam 29: Relativity100 Questions
Exam 30: Quantum Physics100 Questions
Exam 31: Atomic Physics75 Questions
Exam 32: Nuclear Physics and Nuclear Radiation89 Questions
Select questions type
When a bimetallic strip is heated, the strip will bend toward the side
(Multiple Choice)
4.9/5  (30)
(30)
Gas in a constant-volume gas thermometer registers a pressure of 95.0 kPa at 100°C. Assuming ideal behavior, what is the temperature of this gas when the pressure is 190 kPa?
(Multiple Choice)
4.7/5  (37)
(37)
A person running in place on an exercise machine for 10 minutes uses up 17 Calories. Another person exercises by repeatedly lifting two 2.5-kg weights a distance of 50 cm. How many repetitions of this exercise are equivalent to 10 minutes of running?
(Multiple Choice)
4.8/5  (32)
(32)
Two objects are placed in a room with a temperature of 20°C. Object A has a temperature of 70°C, while object B has a temperature of 140°C. They have the same emissivity, but different areas. What should the ratio of the area of object A to that of object B be if the net power emitted by both objects is the same?
(Multiple Choice)
4.9/5  (43)
(43)
The type of heat transfer that occurs between warm food and the air in the room is principally
(Multiple Choice)
4.8/5  (35)
(35)
330 g of water at 45°C are poured into an 855 g aluminum container with an initial temperature of 10°C. The specific heat of aluminum is 900 J/(kg·K). What is the final temperature of the system, assuming no heat is exchanged with the surroundings?
(Multiple Choice)
4.9/5  (33)
(33)
A double-paned window consists of two 60.0 cm × 100 cm glass panes, 2.00 mm thick, separated by a 1.50 cm layer of still air. 25.0 W of heat flow through the window. What is the temperature difference between the indoors and outdoors? The thermal conductivity of glass is 0.840 W/(m·K), and that of air is 0.0234 W/(m·K).
(Multiple Choice)
4.7/5  (39)
(39)
A jogger is running outdoors on a cold day when the temperature is -20.0°C. Each time she breathes, she inhales 0.00450 m3 of air. She is breathing at the rate of 25 breaths per minute. How much heat does she lose from breathing during 20.0 minutes of jogging if the air in her lungs is heated to body temperature (37°C) before it is exhaled? The specific heat of air is 1020 J/(kg∙K) and the density of air is 1.29 kg/m3.
(Multiple Choice)
4.9/5  (36)
(36)
Two metal rods, one silver and the other gold, are attached to each other. The free end of the silver rod is connected to a steam chamber, with a temperature of 100°C, and the free end of the gold rod to an ice water bath, with a temperature of 0°C. The rods are 5.0 cm long and have a square cross-section, 2.0 cm on a side. What is the temperature at the point where the two rods are in contact with one another? The thermal conductivity of silver is 417 W/(m·K) and that of gold is 291 W/(m·K) No heat is exchanged between the rods and the surroundings, except at the ends.
(Multiple Choice)
4.8/5  (27)
(27)
The process whereby heat flows by the mass movement of molecules from one place to another is referred to as
(Multiple Choice)
4.9/5  (32)
(32)
A camper is about to drink his morning coffee. He pours 400 grams of coffee, initially at 75.0°C into a 250-g aluminum cup, initially at 16.0°C. What is the equilibrium temperature of the coffee-cup system, assuming no heat is lost to the surroundings? The specific heat of aluminum is 900 J/(kg·K). Assume that the specific heat of coffee is the same as the specific heat of water.
(Multiple Choice)
4.9/5  (35)
(35)
FIGURE 16-3 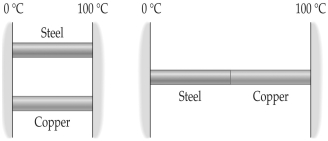 -Two metal rods are to be used to conduct heat from a region at 100°C to a region at 0°C as shown in Figure 16-3. The rods can be placed in parallel, as shown on the left, or in series, as on the right. The heat conducted in the series arrangement is
-Two metal rods are to be used to conduct heat from a region at 100°C to a region at 0°C as shown in Figure 16-3. The rods can be placed in parallel, as shown on the left, or in series, as on the right. The heat conducted in the series arrangement is
(Multiple Choice)
4.8/5  (33)
(33)
Two identical objects are placed in a room with a temperature of 20°C. Object A has a temperature of 50°C, while object B has a temperature of 90°C. What is the ratio of the net power emitted by object B to that emitted by object A?
(Multiple Choice)
4.8/5  (31)
(31)
The thermal conductivity of concrete is 0.80 W/m-C° and the thermal conductivity of wood is 0.10 W/m-C°. How thick would a solid concrete wall have to be in order to have the same rate of flow through it as an 8.0 cm thick wall made of solid wood?
(Assume both walls have the same surface area.)
(Short Answer)
4.8/5  (37)
(37)
The coefficient of linear expansion of aluminum is 24 × 10-6 K-1 and the coefficient of volume expansion of olive oil is 0.68 × 10-3 K-1. A novice cook, in preparation for deep-frying some potatoes fills a 1.00-liter aluminum pot to the brim and heats the oil and the pot from an initial temperature of 15°C to 190°C. To his consternation some olive oil spills over the top. How much?
(Multiple Choice)
4.7/5  (42)
(42)
When you walk barefoot in a room, the floor feels cooler walking on a tile floor as compared to a wooden floor because
(Multiple Choice)
4.9/5  (37)
(37)
A person tries to heat up her bath water by adding 5.0 L of water at 80°C to 60 L of water at 30°C. What is the final temperature of the water?
(Short Answer)
4.9/5  (42)
(42)
If a thermometer measures the temperature of two objects as being equal, you can conclude that if the objects are placed in thermal contact, no heat will flow between them.
(True/False)
4.8/5  (31)
(31)
Betelgeuse is a star in the constellation Orion. It radiates heat at the rate of 3.00 × 1030 W and has a surface temperature of 3000 K. Assuming that it is a perfect emitter, what is the radius of Betelgeuse? The Stefan-Boltzmann constant is 5.67 x 10-8 W/(m2·K4).
(Multiple Choice)
4.8/5  (30)
(30)
A solid concrete wall 4.0 m by 2.4 m and 30 cm thick, with a thermal conductivity of 1.3 W/(m∙K), separates a basement at 18°C from the ground outside at 6°C. How much heat flows through the wall in one hour?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 61 - 80 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)