Exam 16: Temperature and Heat
Exam 1: Introduction to Physics100 Questions
Exam 2: One-Dimensional Kinematics112 Questions
Exam 3: Vectors in Physics82 Questions
Exam 4: Two-Dimensional Kinematics95 Questions
Exam 5: Newtons Laws of Motion101 Questions
Exam 6: Applications of Newtons Laws105 Questions
Exam 7: Work and Kinetic Energy92 Questions
Exam 8: Potential Energy and Conservation of Energy99 Questions
Exam 9: Linear Momentum and Collisions102 Questions
Exam 10: Rotational Kinematics and Energy102 Questions
Exam 11: Rotational Dynamics and Static Equilibrium97 Questions
Exam 12: Gravity94 Questions
Exam 13: Oscillations About Equilibrium102 Questions
Exam 14: Waves and Sound104 Questions
Exam 15: Fluids107 Questions
Exam 16: Temperature and Heat103 Questions
Exam 17: Phases and Phase Changes100 Questions
Exam 18: The Laws of Thermodynamics97 Questions
Exam 19: Electric Charges, Forces, and Fields88 Questions
Exam 20: Electric Potential and Electric Potential Energy99 Questions
Exam 21: Electric Current and Direct-Current Circuits99 Questions
Exam 22: Magnetism101 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction99 Questions
Exam 24: Alternating-Current Circuits93 Questions
Exam 25: Electromagnetic Waves90 Questions
Exam 26: Geometrical Optics92 Questions
Exam 27: Optical Instruments102 Questions
Exam 28: Physical Optics: Interference and Diffraction93 Questions
Exam 29: Relativity100 Questions
Exam 30: Quantum Physics100 Questions
Exam 31: Atomic Physics75 Questions
Exam 32: Nuclear Physics and Nuclear Radiation89 Questions
Select questions type
If you double the thickness of a wall built from a homogeneous material, the rate of heat loss for a given temperature difference across the thickness will
(Multiple Choice)
4.8/5  (29)
(29)
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper; the aluminum and the copper are in thermal contact. The specific heat capacity of aluminum is more than double that of copper. Which object experiences the greater temperature change during the time the system takes to reach thermal equilibrium?
(Multiple Choice)
4.9/5  (40)
(40)
A 400-g piece of metal at 100°C is dropped into a 100-g aluminum cup containing 500 g of water at 15°C. The final temperature of the system is 40°C. What is the specific heat of the metal, assuming no heat is exchanged with the surroundings? The specific heat of aluminum is 900 J/(kg∙K)
(Multiple Choice)
4.9/5  (31)
(31)
Nitrogen boils at -196°C. What is the corresponding temperature in the Fahrenheit scale?
(Multiple Choice)
4.8/5  (40)
(40)
How much heat is required to raise the temperature of a 225-g lead ball from 15.0°C to 25.0°C? The specific heat of lead is 128 J/(kg·K).
(Multiple Choice)
4.7/5  (40)
(40)
A wall consists of 2 layers of material:
brick in front of an equal thickness of wood. If the respective thermal conductivities of the brick and wood are 1.3 J/m-s-C and 0.15 J/m-s-C, how does the temperature gradient across the wood compare to the gradient across the brick?
(Short Answer)
4.8/5  (30)
(30)
550 g of water at 105°C are poured into an 855 g aluminum container with an initial temperature of 11°C. The specific heat of aluminum is 900 J/(kg·K). How much heat flows from the water to the aluminum, assuming no heat is exchanged with the surroundings?
(Multiple Choice)
4.7/5  (44)
(44)
The temperature in a room is 77°F. What is the corresponding temperature in the Celsius scale?
(Multiple Choice)
4.7/5  (31)
(31)
Convective heat transfer can only occur if fluids mediate the energy transfer.
(True/False)
4.9/5  (43)
(43)
A camper carries her drinks in an ice chest made of styrofoam. The chest is 40 cm × 45 cm × 70 cm, and the walls and lid are 2.5 cm thick. The contents of the chest are ice, water, cans of soft drinks, and other food, all of which are in thermal equilibrium with the ice water. The temperature just outside the ice chest is 35°C. What is the rate of heat flow through the walls of the box? The thermal conductivity of styrofoam is 0.010 W/(m·K).
(Multiple Choice)
4.7/5  (34)
(34)
Conductive heat transfer can only occur if solids mediate the energy transfer.
(True/False)
4.9/5  (37)
(37)
An industrial fabrication process stamps out thin metal pieces, each in the shape of a square with a circular hole in the middle, from a large thin sheet. These pieces are mounted in a large machine. As these pieces heat up during regular machine operation, the diameter of the circular hole will
(Multiple Choice)
4.8/5  (42)
(42)
FIGURE 16-3 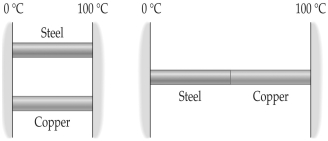 -The process whereby heat flows in the absence of any medium is referred to as
-The process whereby heat flows in the absence of any medium is referred to as
(Multiple Choice)
4.8/5  (33)
(33)
The process whereby heat flows by means of molecular collisions is referred to as
(Multiple Choice)
4.9/5  (38)
(38)
The amount of heat that flows through a rod whose ends are held at different temperatures is proportional to the area of the rod.
(True/False)
4.9/5  (38)
(38)
Gallium boils at 2205°C. What is the corresponding temperature in the Fahrenheit scale?
(Multiple Choice)
5.0/5  (33)
(33)
A carpenter is driving a nail into a board. The 1.00-kg hammer is moving at 8.50 m/s when it strikes the nail. Assume that half of the kinetic energy of the hammer is transformed into heat in the 15.0-g steel nail and does not flow out. What is the increase in temperature of the nail after the three blows that the carpenter needs to drive the nail in completely? The specific heat of steel is 448 J/(kg·K).
(Multiple Choice)
4.8/5  (43)
(43)
The radius of the Sun is 6.95 × 108 m. It radiates heat at the rate of 5.32 x 1026 W. Assuming that it is a perfect emitter, what is the temperature of the surface of the Sun? The Stefan-Boltzmann constant is 5.67 x 10-8 W/(m2·K4).
(Multiple Choice)
4.9/5  (36)
(36)
An aluminum electric tea kettle has a mass of 500 g. It has a 500-W heating coil. How long will it take to heat up 1.0 kg of water from 18°C to 98°C in the tea kettle? The specific heat of aluminum is 900 J/(kg·K).
(Multiple Choice)
4.8/5  (35)
(35)
Showing 81 - 100 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)