Exam 32: Nuclear Physics and Nuclear Radiation
Exam 1: Introduction to Physics100 Questions
Exam 2: One-Dimensional Kinematics112 Questions
Exam 3: Vectors in Physics82 Questions
Exam 4: Two-Dimensional Kinematics95 Questions
Exam 5: Newtons Laws of Motion101 Questions
Exam 6: Applications of Newtons Laws105 Questions
Exam 7: Work and Kinetic Energy92 Questions
Exam 8: Potential Energy and Conservation of Energy99 Questions
Exam 9: Linear Momentum and Collisions102 Questions
Exam 10: Rotational Kinematics and Energy102 Questions
Exam 11: Rotational Dynamics and Static Equilibrium97 Questions
Exam 12: Gravity94 Questions
Exam 13: Oscillations About Equilibrium102 Questions
Exam 14: Waves and Sound104 Questions
Exam 15: Fluids107 Questions
Exam 16: Temperature and Heat103 Questions
Exam 17: Phases and Phase Changes100 Questions
Exam 18: The Laws of Thermodynamics97 Questions
Exam 19: Electric Charges, Forces, and Fields88 Questions
Exam 20: Electric Potential and Electric Potential Energy99 Questions
Exam 21: Electric Current and Direct-Current Circuits99 Questions
Exam 22: Magnetism101 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction99 Questions
Exam 24: Alternating-Current Circuits93 Questions
Exam 25: Electromagnetic Waves90 Questions
Exam 26: Geometrical Optics92 Questions
Exam 27: Optical Instruments102 Questions
Exam 28: Physical Optics: Interference and Diffraction93 Questions
Exam 29: Relativity100 Questions
Exam 30: Quantum Physics100 Questions
Exam 31: Atomic Physics75 Questions
Exam 32: Nuclear Physics and Nuclear Radiation89 Questions
Select questions type
Photons associated with the magnetic field used in magnetic resonance imaging have considerably less energy than the photons used in CT scans.
(True/False)
4.8/5  (28)
(28)
What is the approximate nuclear radius of an isotope of sodium with 11 protons and 12 neutrons?
(Short Answer)
4.9/5  (33)
(33)
If a nuclide has a decay constant of 0.765 × 10-6 s-1 and contains 3.0 × 1017 nuclei
(a) what is the half life?
(b) what is its initial activity?
(c) what is the activity after 12. days?
(Short Answer)
4.7/5  (37)
(37)
The nuclei of different isotopes of a given element have the same number of
(Multiple Choice)
4.9/5  (36)
(36)
Polonium-215 alpha decays with a half life of 1.8 ms.
(a) How much is left after 1. second?
(b) What is the daughter nucleus?
(Essay)
4.8/5  (39)
(39)
A stationary plutonium-239 nucleus decays into a uranium-235 nucleus plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 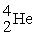 , 4.002603 u;
, 4.002603 u; 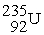 , 235.043924 u; what is the kinetic energy of the
, 235.043924 u; what is the kinetic energy of the 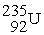 nucleus?
1 u =931.494 MeV/c2.
nucleus?
1 u =931.494 MeV/c2.
(Multiple Choice)
4.9/5  (29)
(29)
Rutherfordium-261 has a half-life of 1.08 min. How long will it take for a sample of rutherfordium to lose one-third of its nuclei?
(Multiple Choice)
4.7/5  (31)
(31)
What are the mass number and the atomic number for each of the following:
(a) beta+?
(b) beta-?
(Short Answer)
4.9/5  (36)
(36)
The symbol for deuterium is 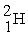 . How many protons are there in a deuterium nucleus?
. How many protons are there in a deuterium nucleus?
(Short Answer)
5.0/5  (32)
(32)
What type of scan is used to find the areas of the body where glucose metabolism is most intense?
(Multiple Choice)
4.9/5  (31)
(31)
A fusion reaction works because the binding energy of the resulting nucleus
(Multiple Choice)
4.9/5  (38)
(38)
The radius of a nucleus is proportional to what power of the mass number?
(Multiple Choice)
4.9/5  (26)
(26)
One mole of Strontium-90 is decaying with a half life of 28. years. After 61. years, how much Strontium is left and what is its activity?
(Essay)
4.8/5  (30)
(30)
A fusion reaction generates energy by combining lighter nuclei into a heavier nucleus, plus additional particles.
(True/False)
5.0/5  (40)
(40)
Showing 41 - 60 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)