Exam 32: Nuclear Physics and Nuclear Radiation
Exam 1: Introduction to Physics100 Questions
Exam 2: One-Dimensional Kinematics112 Questions
Exam 3: Vectors in Physics82 Questions
Exam 4: Two-Dimensional Kinematics95 Questions
Exam 5: Newtons Laws of Motion101 Questions
Exam 6: Applications of Newtons Laws105 Questions
Exam 7: Work and Kinetic Energy92 Questions
Exam 8: Potential Energy and Conservation of Energy99 Questions
Exam 9: Linear Momentum and Collisions102 Questions
Exam 10: Rotational Kinematics and Energy102 Questions
Exam 11: Rotational Dynamics and Static Equilibrium97 Questions
Exam 12: Gravity94 Questions
Exam 13: Oscillations About Equilibrium102 Questions
Exam 14: Waves and Sound104 Questions
Exam 15: Fluids107 Questions
Exam 16: Temperature and Heat103 Questions
Exam 17: Phases and Phase Changes100 Questions
Exam 18: The Laws of Thermodynamics97 Questions
Exam 19: Electric Charges, Forces, and Fields88 Questions
Exam 20: Electric Potential and Electric Potential Energy99 Questions
Exam 21: Electric Current and Direct-Current Circuits99 Questions
Exam 22: Magnetism101 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction99 Questions
Exam 24: Alternating-Current Circuits93 Questions
Exam 25: Electromagnetic Waves90 Questions
Exam 26: Geometrical Optics92 Questions
Exam 27: Optical Instruments102 Questions
Exam 28: Physical Optics: Interference and Diffraction93 Questions
Exam 29: Relativity100 Questions
Exam 30: Quantum Physics100 Questions
Exam 31: Atomic Physics75 Questions
Exam 32: Nuclear Physics and Nuclear Radiation89 Questions
Select questions type
Fermium-253 has a half-life of 3.00 days. A sample of fermium has 7.37 × 107 nuclei. How long will it take for there to be only 3.36 × 106 fermium nuclei in this sample?
(Multiple Choice)
4.9/5  (33)
(33)
What are the mass number and the atomic number for each of the following:
(a) proton?
(b) neutron?
(c) alpha particle?
(Short Answer)
4.9/5  (36)
(36)
One of the particle groups listed below includes two other groups on the list. Which group is it?
(Multiple Choice)
4.8/5  (33)
(33)
Carbon-14 has a half-life of 5730 years. A sample of wood has been recovered by an archaeologist. The sample is sent to a laboratory, where it is determined that the activity of the sample is 0.144 Bq/g. By comparing this activity with the activity of living organic matter, 0.230 Bq/g, the scientist determines how old the wood sample is, or more precisely, when the tree that the sample came from died. How old is the sample of wood?
(Multiple Choice)
4.8/5  (38)
(38)
An oxygen-15 nucleus ( 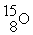 ) decays by emitting a β+ ray and one other particle. What is the other particle?
) decays by emitting a β+ ray and one other particle. What is the other particle?
(Multiple Choice)
4.9/5  (35)
(35)
When an unstable nucleus decays by emitting an alpha particle, the atomic mass number of the nucleus
(Multiple Choice)
4.9/5  (38)
(38)
In beta minus decay, the number of protons in the nucleus is
(Multiple Choice)
4.8/5  (26)
(26)
The neutral deuterium atom, 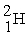 , has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the
, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the 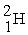 nucleus? 1 u = 931.494 MeV/c2.
nucleus? 1 u = 931.494 MeV/c2.
(Multiple Choice)
4.8/5  (35)
(35)
The density of a nucleus (to a reasonable approximation) is independent of the mass number.
(True/False)
4.8/5  (26)
(26)
Scientists hope that some day they will be able to produce a theory which will combine the strong force, the weak nuclear force, and electromagnetism. This theory has been called
(Multiple Choice)
4.7/5  (30)
(30)
If the "half life" for Radium-226 is 1600. years, how long is the "quarter life"?
(Essay)
5.0/5  (35)
(35)
Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 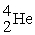 , 4.002603 u;
, 4.002603 u; 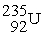 , 235.043924 u; what is the mass value of
, 235.043924 u; what is the mass value of 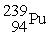 ?
1 u = 931.494 MeV/c2.
?
1 u = 931.494 MeV/c2.
(Multiple Choice)
4.9/5  (28)
(28)
What is the missing term in this fission reaction: 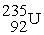 +
+ 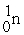 →
→ 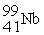 + ?
+ 4
+ ?
+ 4 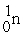
(Multiple Choice)
4.9/5  (31)
(31)
The number of radioactive nuclei in a particular sample decreases to one-eighth of its original number in 9 days. What is the half-life of these nuclei?
(Multiple Choice)
4.9/5  (25)
(25)
Radium-226 decays into radon-222 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are:
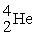 , 4.002603 u;
, 4.002603 u; 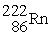 , 222.017570 u;
, 222.017570 u; 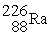 , 226.025402 u.
, 226.025402 u.
(Multiple Choice)
4.8/5  (37)
(37)
If a 2.0-MeV neutron released in a fission reaction loses half its energy in each moderator collision, how may collisions are needed to bring it to (1/25) eV?
(Multiple Choice)
4.8/5  (32)
(32)
A radioactive substance containing 4.00 × 1016 unstable atoms has a half-life of 2.0 days. What is its activity (in curies) after 1 day?
(Short Answer)
4.8/5  (35)
(35)
Showing 21 - 40 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)