Exam 13: The Atomic Nucleus and Radioactivity
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
The alpha particle has twice the electric charge of the beta particle but deflects less than the beta in a magnetic field because it
(Multiple Choice)
4.8/5  (32)
(32)
Why does plutonium not occur in appreciable amounts in natural ore deposits?
(Multiple Choice)
4.8/5  (25)
(25)
Why are smaller nuclei such as carbon-14 often radioactive? (carbon-12 is the most common and most stable form of carbon.)
(Multiple Choice)
5.0/5  (40)
(40)
To predict the approximate energy release of either a fission or a fusion reaction,explain how a physicist uses a table of nuclear masses and the equation E = m .
(Multiple Choice)
4.9/5  (31)
(31)
What is the main technical difficulty in dealing with fusion reactions?
(Multiple Choice)
4.8/5  (41)
(41)
How many alpha particles are emitted in the series of radioactive decay events from a U-238 nucleus to a Pb-206 nucleus?
(Multiple Choice)
4.8/5  (39)
(39)
If three samples have the half-lives listed below,which sample would remain radioactive the longest?
(Multiple Choice)
4.9/5  (35)
(35)
Which process would release energy from gold,fission or fusion? From carbon?
(Multiple Choice)
4.8/5  (34)
(34)
The half-life of carbon-14 is 5730 years.A sample is found to have one-eighth the original amount of carbon-14 in it.How old is the sample?
(Multiple Choice)
4.7/5  (31)
(31)
If uranium were to split into three segments of equal size instead of two,would more energy or less energy be released?
(Multiple Choice)
4.8/5  (31)
(31)
Alpha and beta rays are deflected in opposite directions in a magnetic field because
(Multiple Choice)
4.9/5  (38)
(38)
If a nucleus of 232 90 Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons),what nucleus results?
(Multiple Choice)
4.9/5  (31)
(31)
Which are older,the atoms in the body of an elderly person or those in the body of a baby?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following does not describe a nuclear fission reaction?
(Multiple Choice)
4.9/5  (36)
(36)
The image below shows a beam of radiation passing between two electrically charged plates. 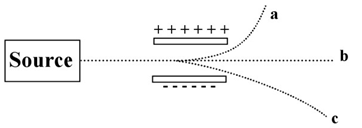 -Which of the beams is actually composed of particles?
-Which of the beams is actually composed of particles?
(Multiple Choice)
4.9/5  (28)
(28)
If a material has a half-life of 24 hours,how long do you have to wait until the amount of radioisotope is 1/4 its original amount?
(Multiple Choice)
4.8/5  (38)
(38)
What are some of the optimistic worldwide changes that may follow the advent of successful fusion reactors?
(Multiple Choice)
4.8/5  (36)
(36)
What evidence supports the contention that the strong nuclear force is stronger than the electrical interaction at short inter-nuclear distances?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)