Exam 13: The Atomic Nucleus and Radioactivity
Exam 1: Patterns of Motion and Equilibrium126 Questions
Exam 2: Newtons Laws of Motion127 Questions
Exam 3: Momentum and Energy181 Questions
Exam 4: Gravity, Projectiles, and Satellites147 Questions
Exam 5: Fluid Mechanics148 Questions
Exam 6: Thermal Energy and Thermodynamics113 Questions
Exam 7: Heat Transfer and Change of Phase158 Questions
Exam 8: Static and Current Electricity178 Questions
Exam 9: Magnetism and Electromagnetic Induction130 Questions
Exam 10: Waves and Sound146 Questions
Exam 11: Light163 Questions
Exam 12: Atoms and the Periodic Table137 Questions
Exam 13: The Atomic Nucleus and Radioactivity127 Questions
Exam 14: Elements of Chemistry69 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React120 Questions
Exam 18: Two Classes of Chemical Reactions177 Questions
Exam 19: Organic Compounds96 Questions
Exam 20: Rocks and Minerals169 Questions
Exam 21: Plate Tectonics and Earths Interior181 Questions
Exam 22: Shaping Earths Surface180 Questions
Exam 23: Geologic Time - Reading the Rock Record167 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects188 Questions
Exam 25: Driving Forces of Weather166 Questions
Exam 26: The Solar System108 Questions
Exam 27: Stars and Galaxies107 Questions
Exam 28: The Structure of Space and Time73 Questions
Exam 29: Prologue: The Nature of Science22 Questions
Select questions type
People who work around radioactivity wear film badges to monitor the amount of radiation that reaches their bodies.These badges consist of small pieces of photographic film enclosed in a light-proof wrapper.What kind of radiation do these devices monitor?
(Multiple Choice)
4.8/5  (46)
(46)
Uranium-235 releases an average of 2.5 neutrons per fission,while plutonium-239 releases an average of 2.7 neutrons per fission.Which of these elements might you therefore expect to have the smaller critical mass?
(Multiple Choice)
4.9/5  (39)
(39)
Which type of radiation is being emitted in the following incomplete nuclear equation? 214 82 Pb ? 214 83 Bi + ??
(Multiple Choice)
5.0/5  (30)
(30)
The image below shows a beam of radiation passing between two electrically charged plates. 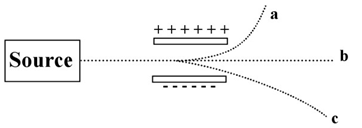 -Which of the beams is due to an energetic light wave?
-Which of the beams is due to an energetic light wave?
(Multiple Choice)
4.8/5  (46)
(46)
Which of the following nuclear equations correctly describes beta emission?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following statements best describes the role of neutrons in the nucleus?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following statements best describes why a large nucleus is more likely to undergo radioactive decay?
(Multiple Choice)
5.0/5  (33)
(33)
Why would you expect alpha particles to be less able to penetrate materials than beta particles of the same kinetic energy?
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following elements is the most stable from a nuclear point of view?
(Multiple Choice)
4.9/5  (44)
(44)
Why is carbon better than lead as a moderator in nuclear reactors?
(Multiple Choice)
4.8/5  (28)
(28)
Why is the combination of two protons and two neutrons stable,but two protons and one neutron is not?
(Multiple Choice)
4.8/5  (40)
(40)
Why is the carbon-14 dating not accurate for estimating the age of materials more than 50,000 years old?
(Multiple Choice)
4.8/5  (34)
(34)
Which type of radiation-alpha,beta,or gamma-results in the greatest change in atomic mass number?
(Multiple Choice)
4.7/5  (40)
(40)
In bombarding atomic nuclei with proton "bullets," the protons must be accelerated to high energies to make contact with the target nuclei
(Multiple Choice)
4.8/5  (25)
(25)
If a nucleus of 232 90 Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons),what nucleus results?
(Multiple Choice)
4.9/5  (41)
(41)
If a fusion reaction produces no appreciable radioactive isotopes,why does a hydrogen bomb produce significant radioactive fallout?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 81 - 100 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)