Exam 7: The Electronic Structure of Atoms
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
Which ground-state atom has an electron configuration described by the following orbital diagram? 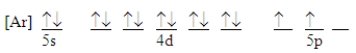
(Multiple Choice)
4.8/5  (27)
(27)
The longest wavelength of light that causes electrons to be ejected from the surface of a copper plate is 243 nm. What is the maximum velocity of the electrons ejected when light of wavelength 200. nm shines on a copper plate?
(Multiple Choice)
4.8/5  (34)
(34)
What is the energy in joules of a mole of photons associated with red light of wavelength 7.00 * 102 nm?
(Multiple Choice)
4.9/5  (36)
(36)
A possible set of quantum numbers for the last electron added to complete an atom of germanium (Ge)in its ground state is 
(Multiple Choice)
5.0/5  (40)
(40)
Each shell (principal energy level)of quantum number n contains n subshells.
(True/False)
4.7/5  (26)
(26)
Which element has the following ground-state electron configuration? [Kr]5s24d105p2
(Multiple Choice)
4.7/5  (40)
(40)
If a hydrogen atom and a helium atom are traveling at the same speed,
(Multiple Choice)
4.9/5  (36)
(36)
In an electron microscope, electrons are accelerated to great velocities. Calculate the wavelength of an electron traveling with a velocity of 7.0 * 103 kilometers per second. The mass of an electron is 9.1 * 10-28 g.
(Multiple Choice)
4.9/5  (37)
(37)
The Bohr model of the hydrogen atom found its greatest support in experimental work on the photoelectric effect.
(True/False)
4.8/5  (35)
(35)
How many electrons in a ground-state tellurium atom are in orbitals labeled by l = 1?
(Multiple Choice)
4.8/5  (41)
(41)
Lanthanide (or rare earth elements)have atoms or ions with partially filled
(Multiple Choice)
4.8/5  (35)
(35)
What is the wavelength of a ball bearing with a mass of 10.0 g, and a velocity of 10.0 cm/s?
(Essay)
5.0/5  (31)
(31)
Rank the following types of electromagnetic radiation from lowest energy to highest energy: infrared, microwave, radio waves, gamma rays, visible, and ultraviolet.
(Essay)
4.9/5  (39)
(39)
The maximum number of electrons that can occupy an energy level described by the principal quantum number, n, is
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following sets of quantum numbers is not possible? 
(Multiple Choice)
4.7/5  (35)
(35)
Which element has the following ground-state electron configuration? [Ar]4s23d104p5
(Multiple Choice)
4.9/5  (37)
(37)
The ground-state electron configuration for an atom of indium is
(Multiple Choice)
4.9/5  (34)
(34)
For all atoms of the same element, the 2s orbital is larger than the 1s orbital.
(True/False)
4.8/5  (44)
(44)
Showing 41 - 60 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)