Exam 7: The Electronic Structure of Atoms
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
A ground-state chromium atom has how many unpaired electrons?
(Multiple Choice)
4.8/5  (47)
(47)
Calculate the wavelength, in nanometers, of the light emitted by a hydrogen atom when its electron falls from the n = 7 to the n = 4 principal energy level. Recall that the energy levels of the H atom are given by En = -2.18 * 10-18 J(1/n2)
(Multiple Choice)
4.8/5  (33)
(33)
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 6 to the n = 3 principal energy level. Recall that for hydrogen En = -2.18 * 10-18 J(1/n2).
(Multiple Choice)
4.7/5  (39)
(39)
A ground-state atom of iron has ___ unpaired electrons and is _____.
(Multiple Choice)
4.9/5  (29)
(29)
A photon is roughly 1800 times more massive than an electron. If a proton and an electron have the same kinetic energy,
(Multiple Choice)
4.9/5  (37)
(37)
A photovoltaic cell converts light into electrical energy. Suppose a certain photovoltaic cell is only 63.5% efficient, in other words, that 63.5% of the light energy is ultimately recovered. If the energy output of this cell is used to heat water, how many 520 nm photons must be absorbed by the photovoltaic cell in order to heat 10.0 g of water from 20.0°C to 30.0°?
(Essay)
4.9/5  (38)
(38)
Which one of the following sets of quantum numbers is not possible? 
(Multiple Choice)
4.8/5  (38)
(38)
Which ground-state atom has an electron configuration described by the following orbital diagram? 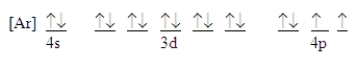
(Multiple Choice)
4.7/5  (34)
(34)
The electron in a hydrogen atom falls from an excited energy level to the ground state in two steps, causing the emission of photons with wavelengths of 1870 and 102.5 nm. What is the quantum number of the initial excited energy level from which the electron falls?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following is the electron configuration of an excited state of an iron atom?
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the wavelength associated with a 20Ne+ ion moving at a velocity of 2.0 * 105 m/s. The atomic mass of Ne-20 is 19.992 amu.
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the wavelength of a neutron that has a velocity of 250 cm/s. (The mass of a neutron = 1.675 * 10-24 g; h = 6.626 * 10-34 J.s)
(Multiple Choice)
4.8/5  (34)
(34)
What is the binding energy (in J/mol or kJ/mol)of an electron in a metal whose threshold frequency for photoelectrons is 2.50 * 1014 /s?
(Multiple Choice)
4.9/5  (30)
(30)
According to de Broglie's equation, the wavelength associated with the motion of a particle increases as the particle mass decreases.
(True/False)
4.9/5  (37)
(37)
The ground-state electron configuration of Cr, Mo, and Ag are exceptions to the Aufbau principle. Which of the following is the electron configuration for Mo?
(Multiple Choice)
4.7/5  (30)
(30)
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level. Recall that for hydrogen En = -2.18 * 10 -18 J(1/n2)
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following is the electron configuration of an excited state of a copper atom?
(Multiple Choice)
4.9/5  (36)
(36)
If we take away two electrons from the outer shell of calcium, it would have the same electron configuration as what element?
(Short Answer)
4.7/5  (40)
(40)
Showing 81 - 100 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)